Whether the cause is related to oil exposure or stress, six primary medical problems have been identified for Early Phase sea otters (Table 5.1). The majority of sea otters arriving at the rehabilitation center during the Early Phase will be heavily or moderately oiled and require immediate cleaning. Special care should be taken when handling animals exhibiting signs of hypothermia or respiratory distress. Unnecessary movement or agitation may induce cardiac arrhythmias and interstitial emphysema associated with these conditions (see below).

Hypothermia
(a) Etiology. For most mammals, hypothermia is defined as a core temperature less than 35°C (95°F) (Knochel, 1985). As body temperature declines, heat production is increased by shivering and heat loss is reduced by decreased peripheral blood flow. If the core temperature drops below 32°C (90°F), shivering ceases, muscle tone increases, and the animal may appear in rigor mortis.
Hypothermia is a serious threat to sea otters during an oil spill. Because oil destroys the insulating quality of the otter’s fur, contamination can result in a rapid decrease of core temperature, especially if the animal remains in the water or is exposed to wind, rain, and cold air temperatures. Oiled otters often forgo feeding to haul out on shore or spend additional time grooming their contaminated fur. The result is a rapid decline in food intake, which can result in hypoglycemia and dehydration, factors that further predispose the otter to hypothermia.
(b) Clinical Manifestations and Diagnosis. The normal rectal temperature of sea otters ranges from 37-39°C (98.6-102°F). During the EVOS, more than 36% of heavily and moderately oiled sea otters arriving at rehabilitation centers were diagnosed as hypothermic. The lowest core body temperature recorded was 29.4°C (85°F) for an otter that arrived cyanotic and unconscious.
Clinical manifestations of hypothermia include locomotor incoordination, disorientation, and lethargy. Peripheral vasoconstriction and shivering are frequent physiological manifestations of mild hypothermia as core temperature declines to 32°C (90°F). At lower core temperatures, hyporeflexia, stupor, cessation of shivering, and muscle rigidity become evident (Knochel, 1985). Left untreated, the hypothermic animal will become unconscious. Reductions in heart rate, blood pressure, peripheral vascular resistance, cardiac output, and central venous pressure occur during severe hypothermia. These cardiovascular changes have a profound effect on organ function and may lead to long-term cellular damage, particularly in metabolically active tissues such as the liver and brain (see Chapter 1). In view of this, the attending veterinarian must consider the possibility of a previous hypothermic event and consequent organ damage for oiled otters, despite the presentation of a normal body temperature during initial examinations.
One of the greatest dangers associated with hypothermia is cardiac arrhythmias, which can result in ventricular fibrillation and death, particularly at core temperatures below 28°C (82°F) (Knochel, 1985). Severe shivering contributes to lactic acid accumulation and resultant metabolic abnormalities. Metabolic acidosis and hyperkalemia may occur if hypothermia is prolonged. The concomitant metabolic imbalance leads to cardiac arrhythmias (Bowen and Bellamy, 1988). Atrial fibrillation and ventricular tachycardia also may occur in cases of severe hypothermia. Physical stimulation predisposes the animal to the development of these arrhythmias. Therefore, handling and physical restraint of the hypothermic otter should be minimized.
Creatine phosphokinase (CPK) increases in the blood during severe hypothermia as a result of cellular damage. We found that serum CPK was elevated in 68% of the oiled otters that died during the EVOS (Appendix 3, Figure F Download PDF). However, CPK also may increase from handling stress and from cardiac and skeletal muscle damage (capture myopathy syndrome) not associated with a hypothermic event (Bossart and Dierauf, 1990). Therefore, CPK should not be considered a diagnostic indicator of cellular damage resulting exclusively from hypothermia.
(c) Treatment. We recommend measuring the core temperature of all sea otters entering the rehabilitation center. Body temperature should also be measured every thirty minutes in anesthetized otters during cleaning. A digital thermometer with a flexible thermocouple probe (Physiotemp, Inc.) should be used. The probe should be inserted at least fifteen cm into the rectum and may be left in place during cleaning and treatment. Glass thermometers are not recommended.
Treatment of the hypothermic animal involves internal and external rewarming and will depend on the state of consciousness and degree of oiling. Passive rewarming at a rate of 0.5°C (1°F) per hour is optimal (Knochel, 1985). Often the core temperature of mildly hypothermic otters will return to normal without additional rewarming therapy the animal is placed in a warm (20°C; 68°F) room. Alert animals may facilitate rewarming by grooming or shivering. The animals should be placed in a dry, well-ventilated cage during this period. If cleaning is delayed, the coat of the otter should be dried with towels or a pet dryer set at room temperature to reduce further heat loss.
Usually, the hypothermic otter is extensively covered with fresh crude oil and is lethargic. In cases of severe hypothermia (core temperature less than 32°C or 90°F) or prolonged hypothermia lasting more than twelve hours, active rewarming is recommended (Zenobl 1980). Laying the hypothermic otter on a recirculating warm water veterinary pad (Aquamatic K Pad, American Hospital Supply) or plastic bags filled with warm water will enhance rewarming. Sedated or lethargic otters thermoregulate poorly and will rapidly gain or lose heat during cleaning (Davis et al., 1988). The veterinarian may use this as an opportunity to slowly rewarm the hypothermic otter by maintaining the wash and rinse water temperatures between 37-40%B0C (98.6-104°F).
Active external rewarming by immersion in warm water is potentially dangerous. External rewarming may cause rewarming shock when lactic acid washed out of previously hypoxic tissues leads severe metabolic acidosis (Knochel, 1985). Earlier studies also describe a paradoxical decrease (after-drop) in core temperature due to peripheral vasodilation associated with warm water immersion in chronic cold-stress patients. It was believed that cold blood returning from the periphery cooled the myocardium and increased the likelihood ventricular fibrillation. However, in more recent investigations, the phenomenon of after-drop in body temperature has been difficult to document. There are insufficient stores of blood in vasoconstricted peripheral areas to cause a decrease in myocardial temperature (Lloyd 1986). Rather than an after-drop in core temperature, the balance between the size of the vascular bed and the circulating blood volume (both of which depend on vasomotor tone and state of hydration) was identified as a critical factor for the survival of humans during rewarming. Rewarming by warm water immersion is currently considered beneficial if cardiovascular and respiratory functions a monitored. Thus, there is growing belief that rewarming by warm water immersion is a fast, effective way of treating hypothermia when the patient is closely monitored. This method is recommended for severely hypothermic animals when core temperature is below 32°C ( 90°F). Water temperature should be 37-40°C (98.6-104°F) and the animal’s vital signs, especially heart rate, must be monitored throughout rewarming.
Several methods of internal rewarming are possible (e.g. high color irrigation, peritoneal dialysis, hemodialysis, intragastric lavage). Most of these techniques are impractical in rehabilitation centers or may cause additional medical complications for animals that are already severely stressed. Rewarming by the administration of warm fluids the safest and preferred method of treatment. Fluid replacement provides additional benefits by improving peripheral circulation and the cardiac output of a hypothermic animal. The fluids should be prewarmed to 37-39°C (98.6-102°F) by passage though a hot water bath or by a bacteriologic incubator. The fluids may be administered subcutaneously or intravenously through the jugular vein or popliteal vein. If the animal is unconscious or sedated, large bore jugular catheters can be used effectively. Lactate-free and potassium-free fluids such as normal saline or a 1-to-1 mixture of 5% dextrose and normal saline (20 ml/kg SQ or IV) are preferred because of the electrolyte and metabolite imbalance of hypothermic patients. A solution containing 10-20% dextrose (10-20 ml/kg IV) is recommended for otters that are hypoglycemic as well as hypothermic. Plasma pH and electrolyte concentrations should be monitored hourly until core temperature returns to 37-39 (98.6-102°F).
During rewarming, sodium-potassium exchange accelerates. As a result, hypokalemia may occur, which can cause cardiac arrhythmias. If cardiac failure occurs, the heart of a hypothermic animal may be unresponsive to lidocaine injections. Cardiopulmonary resuscitation (CPR) and the administration of oxygen should be initiated and continued while core temperature is being raised (Zenoble, 1980). CPR, oxygen administration, intravenous glucose, and warm water immersion were effective in reviving an unconscious, severely hypothermic otter during the EVOS.
Complications following rewarming can include pneumonia, gastric erosions, intravascular erosions, and acute renal tubular necrosis, with pneumonitis the most common problem in human patients (Bowen and Bellamy, 1988). Antibiotic therapy following rewarming along with corticosteroids to combat shock are recommended. Note that the delayed metabolism of drugs in hypothermic animals predisposes them to over medication.
In summary, all animals should be monitored closely during rewarming procedures. Passive rewarming at a rate of 0.5°C (1F) per hour in a room at 20°C (68°F) is the preferred treatment for mildly or moderately hypothermic otters. Severe hypothermia (core temperature less than 32°C or 90°F) should be treated by intravenous or subcutaneous administration of warm, normal saline or a 1-to-1 mixture of normal saline and 5% dextrose. External rewarming by immersion in warm water also is recommended, but the veterinarian or animal care specialist must closely monitor the animal’s vital signs.
Hyperthermia
Panting, dry mucous membranes, lethargy, hind flippers that are warm to the touch, and a core temperature exceeding 39°C (102°F) are manifestations of hyperthermia in sea otters. This condition can occur during transport, anesthesia, or whenever caged otters are placed in a poorly ventilated area warmer than 20°C (68°F) without access to water or ice. Despite the decrease in insulation resulting from oily fur, sea otters easily overheat when out of water. Excessive grooming, inadequate ventilation in transport cages, and hyperactivity associated with handling exacerbate the problem.
Hyperthermia in sea otters is easily prevented by placing the animals in seawater at normal seasonal ocean temperatures. Otters in dry cages should be kept in well ventilated areas at temperatures near 15°C (60°F). The grated bottom of transport cages or critical care cage should be partially covered with chipped ice to enable the otter to cool itself. The otter may also eat the ice which provides additional cooling and helps to prevent dehydration. In extreme cases, where the animal’s core temperature exceeds 40°C (104°F), the hind flipper should be sprayed with water and packed in ice for short periods Because the flippers are well vascularized, cooling them will provide an immediate, short-term benefit to the overheated otter. Care should be taken to avoid localized vasoconstriction of the flippers due to prolonged contact with ice packs.
Petroleum Hydrocarbon Ingestion and Absorption
(a) Etiology. There is considerable confusion concerning the detrimental effects of petroleum hydrocarbon ingestion and absorption. Much of the confusion undoubtedly originates from the fact that oil is a complex mixture of aromatic and aliphatic petroleum hydrocarbons and inorganic compounds, each varying in toxicity. The situation is further complicated by the fact that the chemical composition of oil, and hence its toxicity, changes as it weathers and dissipates. Thus, marine mammals may be exposed to different concentrations of potentially harmful petroleum hydrocarbons during the course of a spill.
Each oil spill will be different, and the effects on wildlife will depend on the type of petroleum hydrocarbons encountered, the degree of weathering, and the duration of exposure. As discussed in Chapter 4, the level of toxicity and the probability of systemic hydrocarbon exposure are greatest during the first weeks of a spill when the oil is fresh and the concentration of aromatic hydrocarbons is highest. In the case of chronic spills, as may occur at marine oil terminals and in harbors, the period of toxicity may be prolonged. Ambient air and water temperatures, weather conditions, and sea state will greatly affect the rate of oil weathering.
Individual petroleum hydrocarbons may be cardiotoxic, hemotoxic, neurotoxic, or hepatotoxic and may induce central nervous system depression (Amdur et al., 1991). As a group, the polycyclic aromatic hydrocarbons (PAHs) are the most toxic. The inhalation of high concentrations of petroleum hydrocarbon vapors can cause excitement, depression, unconsciousness, and death; ingestion can cause severe diarrhea, cardiovascular collapse and organ degeneration (Coppock et al., 1986). The effects of benzene, an aromatic compound commonly found in crude oil, have been examined in studies using laboratory mammals. Exposure to benzene may lead to dose-dependent changes in hematological parameters and lesions in the thymus, bone marrow, spleen, and testes (Ward et al., 1982).
Damage to individual organ systems may occur by direct exposure to petroleum hydrocarbons or secondarily from toxic, metabolic by- products. The lungs, kidneys, and liver are target organs for many toxicants (Klaassen and Rozman, 1991). Petroleum hydrocarbons of high vapor pressure are eliminated through the lungs. The lungs also may reduce hydrocarbons into secondarily toxic metabolites. Because the liver is the site of detoxification and elimination of many compounds, it too is considered vulnerable to the effects of petroleum hydrocarbon absorption. The kidneys are susceptible to damage by toxicants because they receive a large portion (approximately 20%) of the cardiac output, and because tubular secretion and reabsorption may concentrate toxicants within cells. The immune and hematopoietic systems also may be affected.
(b) Clinical Manifestations and Diagnosis. The clinical manifestations of petroleum hydrocarbon toxicosis are difficult to distinguish from other medical problems exhibited by oiled otters. Information about the type of oil and date of the spill will aid the veterinarian in estimating the maximum duration of exposure and the relative toxicity of the contaminant. The degree of external and internal contamination may be assessed by visual examination of the pelt and by blood tests, as described in Chapter 4. Many heavily contaminated otters will spontaneously pass cestodes and acanthocephalids in their feces, providing another indicator of internal oil exposure.
Otters exposed to petroleum hydrocarbons may appear normal during initial examination or display a range of clinical signs including excitability, seizures, CNS depression, lethargy, ataxia, emesis, diarrhea, respiratory distress, and cardiac arrhythmias. The detection of oil in the feces will confirm ingestion. A simple test is to suspend and shake fecal material in water; petroleum products will separate and float to the surface. Hepatocellular enzymes may be elevated (Appendix 3, Figure E Download PDF). Crude oil can be irritating to mucous membranes; corneal ulceration and photophobia may be apparent.
Aspiration pneumonia is a common and serious clinical disorder which can develop in cattle, cats, and dogs exposed to a variety of petroleum products (Hatch, 1988). However, this condition was never observed in sea otters during the EVOS.
(c) Treatment. To prevent further absorption or ingestion of crude oil, sea otters should be moved from the spill area and cleaned with detergent (see Chapter 6). Treatments should focus on delaying absorption and promoting the elimination of ingested oil. The induction of emesis for eliminating ingested petroleum compounds is not recommended due to its limited value when treatment is delayed more than two hours after oil ingestion and the high risk of inhalation pneumonia. Likewise, gastric lavage is not recommended. The oral administration of mineral oil (1 ml/kg) has been used to treat accidental kerosene poisoning in mammals, and may mitigate the absorption of ingested petroleum compounds (Bailey, 1980; Coppock et al., 1986). However, this technique has not been tried on oiled sea otters and it risks aspiration pneumonia associated with vomiting.
Adsorbents such as activated charcoal are often effective in reducing absorption of many ingested toxicants. Several products are available. ToxibanTM (6 ml kg; approximately 120 ml dose) was administered orally to heavily and moderately oiled sea otters during the EVOS. A slurry of activated charcoal and water is administered to sedated sea otters via a syringe connected to a stomach tube, (see section on hypoglycemia for details of tube placement). To reduce stress associated with handling and sedating the otter, we do not recommend a multiple treatment program. Other promising treatments include compounds, such as Questran TM, that bind bile acids in the gastrointestinal tract, thereby preventing hepatic recycling of toxicant: To date, this product has not been used on oiled sea otters.
Oral treatments are less effective against dermal absorption an inhalation of petroleum hydrocarbons. Absorbed compounds may b sequestered in fat or removed by the liver, kidneys, and lungs. The elimination process of some toxicants may be expedited by diuretic peritoneal dialysis, or manipulation of urinary pH (Bailey, 1980). However, we do not recommend these procedures for oiled sea otters. Immunosuppression, metabolic imbalance, impaired renal and hepatic function, and cardiac arrhythmias directly or indirectly associated wit the absorption of petroleum hydrocarbons may complicate these procedures. Most treatments for petroleum hydrocarbon exposure will be limited to supportive care and the mitigation of its effect on individual organ systems.
Injuries to the Respiratory Tract
(a) Etiology. Exposure of the respiratory tract to air borne or blood born petroleum hydrocarbons may lead to pulmonary damage and decreased gas exchange across the alveoli. The specific injury will depend on the route of exposure and can involve the upper and lower respiratory tract. Damage to the gas exchange surfaces will increase the work of breathing.
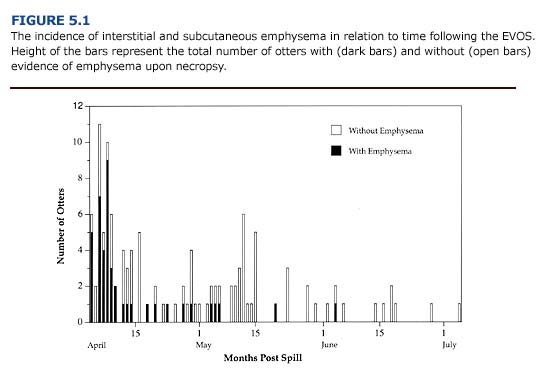
Injuries to the respiratory tract commonly occurred in oiled otters during the first three weeks of the EVOS. Over 75% of oiled sea otters brought to rehabilitation centers during this period showed respirator distress and interstitial emphysema (Figure 5.1; Williams and Davis 1990). The incidence of emphysema during the Early Phase suggests that exposure to volatile petroleum hydrocarbons was a significant contributing factor. Depending on environmental conditions, aromatic hydrocarbons (e.g. benzene, toluene, xylene) will evaporate within days of an oil spill. These are considered the most toxic compounds in crude oil and are known to cause damage to the lungs and mucous membranes of the bronchial airways (Geraci and St. Aubin, 1990).
(b) Clinical Manifestations and Diagnosis. The inhalation or aspiration of toxic compounds produces many pathologic changes in respirator tissues including: 1) bronchospasm, 2) impaired mucociliary clearance 3) mucosal sloughing, 4) atelectasis, and 5) pulmonary edema (Fm row, 1980). Tachypnea, congestion, and the use of accessory respiratory muscles for ventilation are typical clinical manifestations of a respiratory tract injury. The respiratory rate may be accelerated above twenty breaths/minute in oiled sea otters. In other mammals, long-term health problems following exposure to a variety of petroleum hydrocarbon can include aspiration and postinhalation pneumonia. However, his tologic examination indicated that pneumonia did not occur in sea otters that died during the EVOS (Chapter 1).
Respiratory tissue injury in oiled otters ranges in severity from irritation of the nasopharyngeal membranes and rhinitis to interstitial and subcutaneous emphysema. Rhinitis and sinusitis can be persistent problems in oiled sea otters. Clinical manifestations are epistaxis and purulent nasal discharge. During the EVOS, cultures and sensitivity tests revealed the presence of pathogenic E. coli and Proteus. Oral and pharyngeal cavities should be examined for edema and swelling. Discharges should be examined microscopically for cellular debris, red blood cells, neutrophils, and bacteria.
The emphysema observed in oiled sea otters may range in severity and location. The condition is classified as: 1) severe (bullous emphysema in the lungs and interstitial areas), 2) moderate (bullous emphysema throughout the lungs), 3) mild (focal areas of lung damage), and 4) none (no evidence of interstitial or subcutaneous emphysema). Subcutaneous emphysema is characterized by pockets of air below the skin. Small bubbles may be felt subcutaneously in the axillary region of the otters. In severe cases, air pockets can be felt or seen beneath the skin along both sides of the neck, thorax, and along the spine. Postmortem examination of otters that died with this condition during the EVOS revealed that the subcutaneous emphysema arose from ruptured membranes in the lungs. Air escaping from the lungs moved along the mediastinum, through the thoracic inlet and accumulated in subcutaneous tissues. To assess the presence of subcutaneous emphysema, the axillary and chest region of the animal should be palpated during the initial physical examination. Gas bullae may be felt as distortions below the skin and can be heard “popping” (crepitation) when pressed lightly. Roentgenographic examination can confirm this condition, but is of little practical use and subjects the animal to additional stress.
The development of interstitial and subcutaneous emphysema in oiled sea otters is not completely understood. One possible explanation is that chemical irritation of the airways from breathing petroleum hydrocarbon vapors leads to bronchial or laryngeal constriction. Nasal passages may become congested due to inflammation of the mucous membranes. When accompanied by labored breathing, alveolar rupture and bullae formation may ensue. Consequently, the handling of animals with suspected emphysema (i.e. heavily and moderately oiled otters captured during the first weeks of a spill) should be minimized (c) Treatment. The treatment of respiratory injuries is limited and based on methods for mitigating human injuries due to inhalation of toxic substances (Farrow, 1980). Further exposure should be prevented by removing the animal from the spill area and by cleaning contaminate fur. The correlation between the severity of emphysema and presence of volatile components of fresh crude (see Chapter 4) indicates that these measures should occur as soon as possible during the Early Phase of a spill. If the animal is agitated, diazepam (0.2 mg/kg PO or 0.1 mg/kg 1M) may be administered to prevent hyperventilation and to reduce the animal’s activity level and metabolic rate.
Rhinitis and sinusitis, as determined from cultures and sensitivity tests, should be treated with antibiotics. Initially the otters may be placed on emofloxacin (2.5 mg/kg bid IM or PO) for mature otter and amoxicillin (12 mg/kg bid IM) for immature otters. Although antibiotics may decrease the frequency of epistaxis and nasal discharge full recovery may take as long as three months. Respiratory parasites such as nasal mites should be controlled with Ivermectin (50 ug/kg 2 a single dose SQ or PO).
The treatment of interstitial and subcutaneous emphysema is limited to supportive care. In clinical settings, supplemental oxygen has been used to help alleviate respiratory distress. However, its large scale use for oiled sea otters is impractical. Positive pressure ventilation systems may aggravate this condition by inducing the formation (of bullae in alveolar membranes weakened by exposure to petroleum hydrocarbons; positive pressure delivery of oxygen is not recommended. Positive pressure and inhalation anesthetics during cleaning procedures also are contraindicated for otters showing signs of emphysema. Aminophylline (10 mg/kg bid PO, slow release form), bronchodilator, is recommended for any otter exhibiting respirator distress. However, aminophylline is a diuretic and can cause cardiac arrhythmias. Therefore, this treatment is not recommended for animals exhibiting renal insufficiency or hypothermia.
Hypoglycemia
(a) Etiology. Hypoglycemia (plasma glucose less than 60 mg/ dl) in oiled sea otters may result from: 1) inability to feed prior to capture and, reduction in glycogen stores, 2) fasting during capture and transpor1 3) impaired hepatic function or intestinal absorption, or 4) stress and shock. The high metabolic rate of sea otters in general (Costa and Kooyman, 1982), and oiled sea otters in particular (Davis et al., 1988) predisposes these animals to hypoglycemia when food is withheld for several hours. Other factors such as anorexia, hypothermia, and fever also may predispose them to hypoglycemia. Following the EVOS 40% (n = 27) of the otters that died were hypoglycemic (Appendix 3 Figure A Download PDF).
(b) Clinical Manifestations and Diagnosis. Symptoms of hypoglycemic include pulmonary edema, hypothermia, and central nervous system dysfunction (i.e. depression, seizures, muscular weakness, and locomotor incoordination). In severe cases, the animal may become unconscious, and prompt treatment is critical. Plasma glucose concentration should be measured during the initial physical examination of all oiled otters. Reagent strips (Gluco-StixTM, Ames Laboratories) provide a rapid, qualitative measure of blood glucose concentration. This allows the veterinarian to verify the hypoglycemia and initiate emergency treatments. We recommend a subsequent quantitative measurement of plasma glucose as soon as possible. Desktop blood chemistry analyzers (Eastman Kodak, Inc.; Abbot Laboratories) or small digital analyzers for routine blood glucose monitoring in diabetics provide rapid results from small quantities of blood.
(c) Treatment. Hypoglycemia is treated by increasing the otter’s intake of dextrose (glucose) as soon as possible. The voluntary ingestion of dextrose can be achieved by offering chipped ice balls containing a 50% dextrose solution. If the animal is lethargic or semiconscious, administer 10-20% dextrose (10-20 ml/kg IV to effect). The oral administration of 50% dextrose (1 ml/kg) through a stomach tube will provide an immediate but temporary increase in the plasma glucose concentration. In general, the otter will respond to treatment within thirty minutes of fluid administration, but relapses may occur if the underlying dietary, hepatic, or gastrointestinal problems are not resolved. Following initial treatment, hypoglycemic otters should be fed frequently (at least every hour) until stable. If seizures occur after blood glucose is stabilized, diazepam (0.1 mg/kg 1M) should be administered.
Insulin resistance may limit the effectiveness of prolonged glucose administration and carbohydrate feeding to supplement calories in animals that refuse to eat. If an otter can not be encouraged to eat voluntarily, enteral feeding (stomach tube feeding) may be necessary. Animals that will not eat utilize stored fat and tissue protein for metabolic energy. The primary objective of enteral feeding is to prevent the loss of tissue protein by providing nutrients that can be readily digested and absorbed.
During enteral feeding, the otter should be chemically restrained (see Chapter 3), although severely debilitated otters may be physically restrained by experienced handlers. A plastic or wooden dowel should be placed between the premolars, and a stomach tube inserted into the esophagus to a premeasured length (about twenty cm in adult otters). The tube can usually be seen or felt to pass on the left side of the trachea. Proper placement of the tube in the stomach should be tested by listening for bubbling sounds when air is blown into the tube. The enteral diet is injected into the feeding tube with a syringe. After feeding, the tube is sealed while it is withdrawn to prevent leakage of residual fluids into the pharynx.
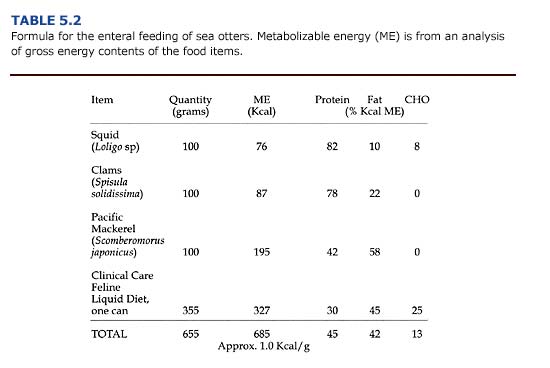
Enteral diets are slurries of common food items blended with a diluent of either liquid enteral (e.g. PedialyteTM) or water. Carnivores require more fat and protein than the enterals manufactured for humans. An example of a slurry suitable for sea otters is shown in Table 5.2. Resting adult sea otters require about 75 kcal/kg/ day in their diet in order to maintain their body weight (Davis et al., 1988); additional food energy would be required for an otter to gain weight. Because of stress caused by orogastric intubation, enteral meals are given only three times daily. Thus, meal size is relatively large. An otter weighing 40 kg requires 3.0 kg (1.0 kg per meal x 3) of the high-energy diet shown in Table 5.2 daily.
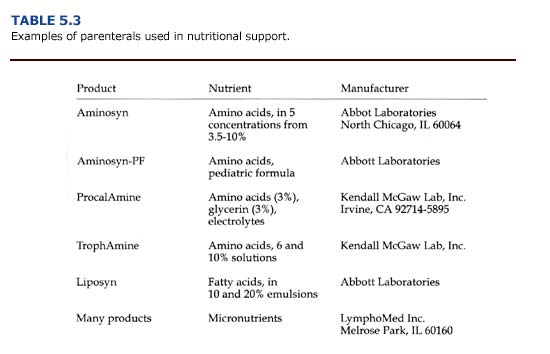
Calories and nutrients given parenterally consist of solutions of glucose, amino acids, and lipid emulsions administered intravenously (Table 5.3). For carnivores, dextrose (25 or 50% solutions), amino acids (3.5-10% solutions), and lipids (10 or 20% emulsions) are combined to provide about 35-45% of calories from carbohydrates, 20-25% of calories from amino acids, and 35-45% of calories from fat. Solutions of vitamins, trace minerals, and electrolytes should be provided as well. Parenteral nutrition is expensive, labor intensive, and risks sepsis. For sea otters, it should be used only for critically ill animals, and the switch to enteral or normal feeding should be made as soon as possible.
Shock
(a) Etiology. All forms of shock are characterized by acute circulatory insufficiency and inadequate capillary perfusion. Hypovolemic shock due to severe blood loss is rare in oiled sea otters, but should be considered in cases of gastrointestinal hemorrhage (see below). Oiled otters that are hypothermic or stressed may also experience shock due to reduced cardiac output or peripheral vasodilatation. A consequence of the reduction in tissue perfusion is localized hypoxic ischemia and impaired cellular function. To compensate for the general reduction in tissue circulation, the body may respond by readjusting blood flow to vital organs such as the heart and brain. At the cellular level, poor tissue perfusion will result in cellular swelling, intracellular acidosis (as a result of elevated lactic acid production), and extracellular acidemia. The onset of these conditions will depend on the animal’s body temperature.
(b) Clinical Manifestations and Diagnosis. It is critical that symptoms of shock be recognized and treated quickly. Primary signs of shock in otters are tachycardia, hyperventilation, depression, muscular weakness, cold hind flippers, and hypotension (poor capillary refill, reduced pulse pressure). Poor peripheral perfusion can be detected by the palpation of cold extremities and observing capillary refill times of mucous membranes. Refill times exceeding two seconds indicate inadequate peripheral perfusion. (c) Treatment. Treatments for shock will be specific for the individual otter and depend on underlying causes. Detailed treatments are complex and are described in detail elsewhere (see for example, Veterinary Pharmacology and Therapeutics, Iowa State University Press; Current Veterinary Therapy, W. B. Saunders Company). For most oiled sea otters, the veterinarian should initiate fluid volume expansion to reestablish adequate tissue perfusion as soon as possible. Administration of normal saline or a 1-to-1 mixture of normal saline and 5% dextrose (20 ml/kg IV) is recommended. Sodium bicarbonate (1 mEq/ kg IV or 50 mg/kg PO) or supplemental dextrose (1 ml/kg of a 50% dextrose solution by stomach tube) may be indicated, if the animal is acidotic or hypoglycemic, respectively. Finally, dexamethasone (1-2 mg/kg/ day 1M) or methylprednisolone (0.06 mg/kg / day 1M or IV) should be administered.
Seizures
(a) Etiology. Seizures in oiled sea otters may be caused by:
1) hypoglycemia,
2) hypothermia or hyperthermia,
3) hepatic encephalopathy,
4) electrolyte imbalances,
5) dehydration,
6) sepsis,
7) exposure to petroleum hydrocarbons, or
8) adverse reaction to anesthetics (i.e. fentanyl).
Periodic seizures in some animals may persist for weeks to months, slowly reducing in frequency and intensity over time.
(b) Clinical Manifestations and Diagnosis. Depending on the cause, seizures or convulsions are characterized by one or all of the following signs: unconsciousness, loss of (flaccid) or excess (clonus to rigidity) muscle tone, changes in the autonomic nervous system (urination, salivation, defecation, vomiting), and behavioral abnormalities (vocalization, pacing) (Parker, 1980).
(c) Treatment. As with shock, the treatment of seizures will depend on the underlying cause(s). For repeated or prolonged seizures, anticonvulsant drugs may be warranted (diazepam, 0.2 mg/kg PO or 0.1 mg/kg 1M). In cases of hypoglycemic seizures, anticonvulsants are not necessary; these animals will respond rapidly to glucose administration (see previous section on Hypoglycemia). Seizures associated with hepatoencephalopathy may be reduced by the oral administration of antibiotics to reduce the number of ammonia-producing bacteria in the bowel (see Hepatic Dysfunction in the next section).

