Sea otters arriving at the rehabilitation center three weeks or more after a spill will usually have light or patchy oil on their fur. Because the oil has weathered and the more toxic components have evaporated or dissipated in the water, fewer medical disorders are found in these animals. Nevertheless, each otter should receive a medical examination and the prophylactic treatments described under Stabilization in Chapter 4. These animals usually are alert and will require sedation if cleaning is necessary. Blood and fecal samples should be taken at this time. Five primary disorders have been identified for sea otters contaminated during the Late Phase of an oil spill (Table 5.1).

Hepatic Dysfunction
(a) Etiology.The liver plays a central role in many essential physiological processes, including the biotransformation and detoxification of a wide variety of endogenous and exogenous substances. During the process of detoxifying petroleum hydrocarbons, the liver plays a protective role and bears the brunt of potential adverse effects from toxins. Consequently, of the four tissues examined, the liver showed the highest concentration of petroleum hydrocarbons in oiled sea otters (see Chapter 1).
Histopathologic examination of liver samples from sea otters that died during the EVOS showed that both cardiovascular insufficiency and toxicosis were factors contributing to cellular damage in this organ (Chapter 1; Lipscomb et al., 1993, 1994). Cardiovascular congestion may have resulted from shock caused by hypothermia, sepsis, or stress. Hypothermia, in particular, may lead to hepatic congestion and lipidosis with consequent regional hypoxia and cellular damage. These studies showed that the effects of a hypothermic event on the liver may persist long after the core body temperature and tissue perfusion have returned to normal levels in oiled sea otters.
(b) Clinical Manifestations and Diagnosis. The clinical manifestations of liver dysfunction are varied and, even when advanced, may consist of nonspecific signs including fatigue, malaise, fever, anorexia, weight loss, nausea, and vomiting (Ockner, 1985). Most of these signs were evident in heavily oiled sea otters during the first two weeks of the EVOS. Although none of these signs are specific for hepatic dysfunction, veterinarians in the rehabilitation center should recognize that oiled sea otters may experience liver damage. Elevations in the serum concentration of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) are good indicators of hepatocellular damage. Fifty-four percent (n = 34) of the sea otters that died following the EVOS had elevated concentrations of ALT, and 61% (n = 41) had elevated concentrations of AST (Appendix 3, Figure E Download PDF). Because the enzyme ALT is found primarily in the liver, an increase in the serum concentration is considered specific for hepatocellular damage in many mammals (Kerr, 1989). The enzyme AST is found in cardiac and skeletal muscle, liver, brain, and other tissues. Thus, an increase in serum AST indicates nonspecific tissue damage. Handling stress may also increase this enzyme in pinnipeds (Medway and Geraci, 1986). Nevertheless, an increase in AST in conjunction with ALT (Figure 5.2) reinforces the diagnosis of hepatic damage. Other clinical indicators of liver damage include an increase in serum bilirubin and alkaline phosphatase, hypoalbuminemia, increased prothrombin time (clotting time), and hypertriglyceridemia.
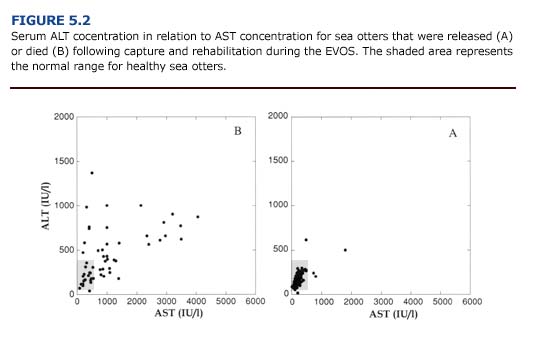
Of the otters necropsied following the EVOS, 30% (n = 41) showed macroscopic evidence of liver damage (friable, discolored, hemorrhagic tissue). Histological examination of the livers showed tissue necrosis, fatty degeneration, hemorrhage, and pericholangitis (inflammation of the tissues that surround the bile ducts). Rather than resulting solely from petroleum hydrocarbon absorption, liver damage in oiled otters has been attributed to several factors, including cardiovascular collapse, hypothermia, shock, renal insufficiency, and sepsis (Lipscomb et al., 1993, 1994).
Hepatic encephalopathy syndrome can be a serious consequence of liver damage. Subjects with this syndrome show abnormal neurological symptoms including myoclonus (shock-like contractions of muscles), hyperactive muscle stretch reflexes, convulsions, facial grimacing, and blinking (Scharschmidt, 1985). Evidence of these neurological abnormalities were observed during the Early Phase of the EVOS. Although the pathogenesis of hepatic encephalopathy is unclear, it may result from toxic materials that are derived from the metabolism of nitrogenous substrate in the gut (Scharschmidt, 1985). Factors that may precipitate hepatic encephalopathy include azotemia (increased BUN), gastrointestinal hemorrhage, infection, a high protein diet, and tissue hypoxia as a result of hypothermia or shock. All of these factors could be present in heavily and moderately oiled sea otters.
(c) Treatment.There is no clinically established way of initiating hepatic regeneration or improving hepatic function; the management of animals with liver damage is largely supportive (Scharschmidt, 1985). Because sedatives and anesthetics are potentially hepatotoxic, physical restraint should be used for cleaning heavily oiled otters. Once the otter is cleaned and clinically stable, all nonessential drugs should be stopped, especially sedatives and potentially hepatotoxic agents.
It is generally recommended that animals with signs of hepatic encephalopathy syndrome be placed on a low protein diet to help reduce the concentration of urea nitrogen in the blood (BUN). Because sea otters normally eat a high protein diet of shellfish, substituting a low protein diet is virtually impossible, unless enteral feeding is considered. The administration of low protein, artificial diets by orogastric intubation may be beneficial for animals with a severely elevated BUN and pronounced signs of hepatic encephalopathy syndrome. Frequent monitoring of blood glucose is necessary, and the administration of a 50% dextrose solution by stomach tube is recommended if the otter becomes hypoglycemic.
Complications associated with liver damage include gastrointestinal bleeding and bacteremia. During the EVOS, oiled sea otters often showed signs of gastrointestinal bleeding throughout the rehabilitation process. Bacteremia generally results from Staphylococcus aureus and E. coli in humans (Scharschmidt, 1985). Prophylactic antibiotic therapy is recommended for otters that are heavily or moderately oiled (see Chapter 4).
Renal Dysfunction.
(a) Etiology. Renal dysfunction may be classified as a prerenal or a primary renal failure. Prerenal dysfunction is associated with decreased renal perfusion as occurs in hypothermic animals. Primary renal failure has many causes including infection, ischemia, and toxemia. Toxic insults, in particular, are a common cause of acute renal dysfunction, usually resulting in irreversible damage to the proximal tubules. However, histopathologic evidence suggests that prerenal failure predominated in oiled otters during the EVOS (see Chapter 1; Lipscomb et al., 1993, 1994).
Acute renal failure (ARF) will result from an abrupt decline in renal function and is characterized by impaired regulation of water and solute balance in the animal. The hallmark of ARF is a decrease in glomerular filtration rate, but the resulting azotemia (increased BUN) may not be evident until severe (75%) renal tubular damage has occurred. Urine volume may be normal or decreased.
No information is available on the susceptibility of sea otters to ARF. However, many of the factors that predispose cats and dogs to ARF are generally present in sea otters during an oil spill. These factors and their probable causes (in parentheses) include:
1) dehydration (inability to feed or anorexia),
2) decreased cardiac output and renal hypoxia (hypothermia and shock),
3) liver damage (hypothermia or toxicant-induced liver damage),
4) sepsis and fever (toxicant-induced immunosuppression and stress), or
5) concurrent use of potentially nephrotoxic drugs (e.g. antibiotics to treat infection).
(b) Clinical Manifestations and Diagnosis. The type and severity of clinical signs for ARF will depend on the kidney’s ability to compensate for the decreased blood flow. Clinical manifestations may include vomiting, diarrhea, depression, anorexia, oliguria, hypothermia, and bradycardia (Thornhill, 1980). Dehydration, electrolyte abnormalities and acid-base imbalance are primary indicators of renal dysfunction. Dehydration occurs when fluid loss (from vomiting, diarrhea, and diuresis) exceeds fluid intake. Decreased food intake will also contribute to the fluid deficit. Loss of skin elasticity, dry mucous membranes and sunken globes indicate dehydration. In severe cases, tachycardi may occur. An increase in packed cell volume and total plasma protein concentration associated with dehydration are also important diagnostic indicators.
Electrolyte abnormalities associated with renal insufficiency have been observed in oiled sea otters, including elevations in serum concentrations of potassium (hyperkalemia), phosphorus (hyperphosphatemia), and chloride (hyperchloremia). Of the adult sea otters that died following the EVOS, 51% (n = 32) had an elevated potassium concentration, 30% (n = 19) had an elevated phosphorus concentration, and 19% (n = 12) had an elevated chloride concentration (Appendix 3, Figures B-D Download PDF). Serum potassium concentration approaching or exceeding 7 mEq/l should be regarded as an emergency; this concentration of extracellular fluid potassium is liable to induce cardiac arrest. Only 5% of the otters that died in the rehabilitation center during the EVOS showed a decrease in serum sodium concentration (hyponatremia). Serum calcium concentrations were normal for surviving oiled otters, as well as those that died (Appendix 3, Figure C Download PDF).
The acid-base balance of oiled otters may be disrupted by impaired renal function or by hypothermia. Although changes in ventilation can compensate for reduced serum bicarbonate during metabolic acidosis, this may not be an option for oiled animals showing poor respiratory function. Decreases in blood pH below 7.0 will cause depression, coma, and hyperkalemia, which in turn can cause cardiac arrhythmias.
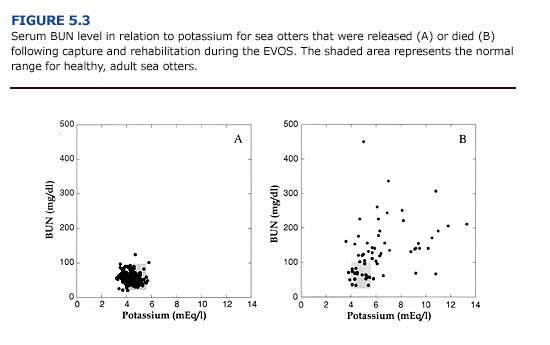
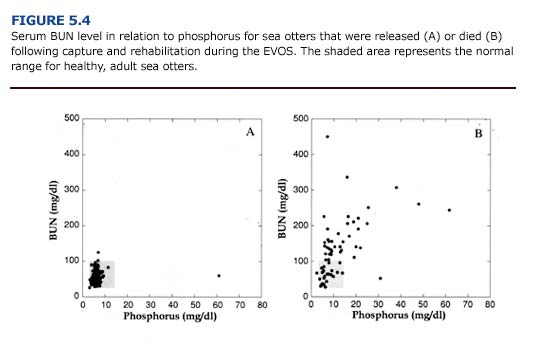
In cases of renal insufficiency, there is a decrease in urea excretion and a consequent elevation in serum urea concentration. Blood urea nitrogen (BUN) levels provide an indication of renal function and may be elevated in oiled animals as a result of dehydration, reduced renal perfusion, or primary renal failure. Because a variety of pathologic conditions may result in increased serum urea concentrations, elevate BUN should not be used as the only prognostic indicator for renal failure (Kerr, 1989). Following the EVOS, 66% (n = 45) of the oiled otters that died had an elevated serum BUN concentration (Append] 3, Figure D). This often coincided with an increase in serum potassium (Figure 5.3) and phosphorus (Figure 5.4). Elevated BUN level usually cause gastrointestinal inflammation and ulceration, which further interferes with feeding and fluid intake in uremic animals.
(c) Treatment. The primary goal in treating renal failure is to restore normal hydration and electrolyte balance until renal blood flow an glomerular filtration are reestablished. A positive response to therapy is indicated by increased urine production and a decrease in serum BUN, potassium, and phosphorus. Because renal failure may occur during the rehabilitation of oiled sea otters, potentially nephrotoxic drugs should be avoided.
To prevent further renal ischemia, dehydration must be treated quickly. Isotonic fluids (normal saline or a 1-to-1 mixture of normal saline and 5% dextrose, 20 ml/kg SQ or IV) should be administered subcutaneously. An intravenous or intraosseous route should be used in cases of severe dehydration in which peripheral perfusion is reduced (Black and Williams, 1993). Although urine production should be measured to properly assess maintenance fluid requirements, this is usually impractical with sea otters. Qualitative indications of urine volume and frequency should be noted on the animal’s record (Appendix 2, Form I Download PDF). Serum electrolyte concentrations, PCV / total solutes, and body weight should be monitored closely to avoid over hydration.
Hyperkalemia can cause cardiac conduction abnormalities and is the most life-threatening electrolyte imbalance that occurs in renal dysfunction. Severe hyperkalemia (serum concentration greater than 7 mEq/l) should be promptly treated with crystalline insulin (2 units/ kg/ day SQ). Alternatively, slow intravenous administration of 1-2 mEq/kg of either sodium bicarbonate or a solution of 5% dextrose is helpful. The former has the added benefit of managing acidosis. The administration of fluids is also important for animals with elevated phosphorus levels. In all cases, treatments for electrolyte or acid-base imbalances should be monitored closely.
When fluid therapy fails to induce diuresis (urine formation), either 10% dextrose (20 ml/kg administered as a slow IV infusion), mannitol (1-2 gm/kg as a 25% solution administered in a slow IV bolus) or furosemide (2 mg/kg 1M) is recommended (Grauer, 1986). These treatments require experienced management and constant monitoring. Whether or not diuresis occurs, qualitative urine formation and serum electrolyte concentrations should be monitored as long as the animal receives maintenance fluids.
Providing daily caloric requirements is an important aspect of managing animals with renal dysfunction. Energy requirements have a higher priority than do protein requirements, although supplementation of essential amino acids will reduce body protein breakdown and reduce urea nitrogen formation. Sea otters may be given cimetidine (5-10 mg/kg tid IM or PO) or sucralfate (0.5-1.0 gm tid PO) to combat gastrointestinal inflammation. However, the use of metoclopramide is contraindicated (T. D. Williams, personal communication). Enrofloxacin (2.5 mg/kg bid 1M or PO) for mature otters and amoxicillin (12 mg/kg bid 1M) for immature otters should be given to prevent or treat sepsis. Dexamethasone (1-2 mg/kg/ day 1M) may be given to counter the symptoms of shock which may accompany renal insufficiency.
Gastrointestinal Disorders
(a) Etiology.Sea otters in the rehabilitation center often exhibit gastrointestinal disorders. These conditions may range in severity from intestinal irritation to life-threatening hemorrhagic gastroenteritis. Possible causes include dietary changes, stress, parasites, uremia, and the ingestion of petroleum hydrocarbons.
(b) Clinical Manifestations and Diagnosis. The clinical signs of gastrointestinal disorders will depend on the location of the condition (stomach, large or small intestines). Vomiting suggests gastric problems. Rectal tenesmus, anal prolapse, and diarrhea indicate colonic and rectal disorders (Palminteri and Ryan, 1981). Oiled sea otters may also exhibit dehydration, oral or rectal bleeding, dyspnea, and abdominal tympany, which are associated with nonspecific gastrointestinal problems.
Melena (dark, tarry stools) is often observed in oiled sea otters during the first weeks of rehabilitation. Unfortunately, oil, blood, squid ink (from ingested squid), and activated charcoal administered to adsorb petroleum hydrocarbons all cause darkened stools. HemocultTM tests are useful for detecting the presence of blood but false positives may result from dietary items containing blood (i.e. whole fish). The presence of oil in fecal material should be determined as described in the preceding section, Petroleum Hydrocarbon Ingestion and Absorption.
(c) Treatment. A treatment regimen will depend on the origin and severity of the gastrointestinal problem. Hemorrhagic enteritis may be fatal within twenty-four hours and requires immediate attention. Treatments include subcutaneous, intravenous and intraosseous administration of fluids and electrolytes to maintain hydration state and acid-base balance. Dexamethasone (1-2 mg/kg/day IM) or methylprednisolone (0.06 mg/kg/ day IM or IV) are recommended (Palminteri and Ryan, 1981). These should be supplemented with broad spectrum antibiotics, Vitamin B-complex, and gastrointestinal motility modifiers (diphenoxylate at 0.1-0.2 mg/kg bid PO or aminopentamide sulfate at 0.1-0.4 mg/kg bid IM, SQ or PO). Successful treatment will result in the cessation of diarrhea and a normal packed cell volume (PCV).
Gastric and intestinal ulcers commonly occur in oiled sea otters undergoing rehabilitation (Chapter 1). The recommended treatment is to reduce stress (excessive handling, noise, and human contact) and to administer cimetidine (5-10 mg/kg tid PO or 10 mg/kg qid IV or IM). However, cimetidine binds cytochrome p450, which may interfere with the hepatic detoxification of petroleum hydrocarbons. Ranitidine (1-4 mg/kg tid PO) or sucralfate (0.5-1.0 gm tid PO), which do not bind cytochrome p450, may be substituted for cimetidine.
A variety of parasites (nematodes, cestodes, and acanthocephalids) commonly occur in wild sea otters. A qualitative assessment of infestation can be obtained by observing parasites in the stools of otters that have ingested oil, or by the microscopic examination of fecal samples for parasite ova. Although not usually lethal in healthy otters, a large parasite load may compromise the recovery of sea otters exposed to oil. Cestodes also may cause vitamin B deficiency. We recommend prophylactic treatment for gastrointestinal parasites in ambulatory otters. Praziquantel (6 mg/kg as a single dose SQ or PO) should be used to treat cestode infestations, and Ivermectin (50 ug/kg as a single dose SQ or PO) should be used for the treatment of nematodes. Supplemental vitamin B is recommended for sea otters during the treatment for parasites.
Anemia
(a) Etiology.Anemia (inadequate circulating red cell mass) in oiled sea otters may result from renal and hepatic dysfunction, inflammation, hemorrhage and petroleum hydrocarbon toxicosis. Because the etiology may vary, oiled sea otters can display both regenerative and nonregenerative types of anemia. Hemolytic anemia, chronic hemorrhage and iron deficiency anemia, and hypoplastic anemia have been reported for oiled wildlife (Williams and Davis, 1990; White, 1991). Chemical toxin-induced anemias may be associated with marrow aplasia, maturational defects, and hemolysis by both direct or immune meditated mechanisms (Keitt, 1985). All forms of anemia decrease tissue oxygenation. To compensate for the reduction in oxygen delivery to tissues, heart rate and cardiac output may increase.
(b) Clinical Manifestations and Diagnosis. Packed cell volume less than 33% (also hemoglobin concentration less than 22.9 g/ dl and a red blood cell count less than 6.5 x 106/ ml), poor tolerance to activity, pale mucous membranes, and tachycardia are common manifestations of anemia. Animals with anemia routinely exhibit depression, weakness, lethargy, and anorexia. Cardiomegaly, resulting from the increased work of the heart in chronically anemic animals, is a common radiographic finding. The anemias are differentiated based on packed cell volume, plasma protein concentration, erythrocyte size and shape, blood hemoglobin concentration, and careful examination of blood smears (Keitt, 1985). Heinz body formation, evidence of erythrocyte abnormalities or damage, and regenerative erythrocyte response are typical hematological findings for oiled mammals and birds. However, Heinz bodies were rare in blood samples from sea otters during the EVOS.
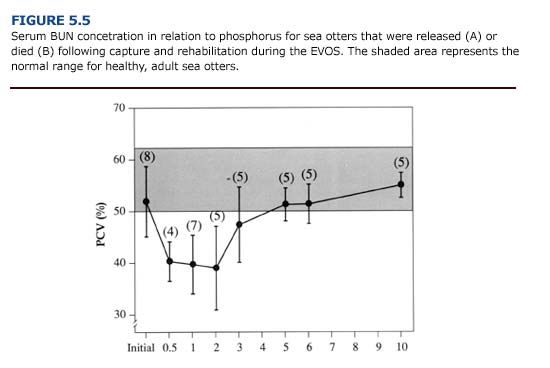
The anemic condition often develops over time and may not be apparent in animals on admission to the rehabilitation center. Heavily oiled otters that die quickly often show normal and even elevated packed cell volumes, red blood cell concentrations and hemoglobin concentration (Appendix 3, Figure G and H Download PDF) due to dehydration. A reduction in circulating red blood cells may develop one to two weeks after capture, especially in heavily oiled sea otters (Figure 5.5). Williams (1990) found that anemia can persist for three to four months following exposure to crude oil.
(c) Treatment. The direct treatment of anemia is of little value until the underlying cause is corrected. For oiled otters, an improvement in hepatic and renal function, elimination of infection and hemorrhages, hepatic detoxification of systemic petroleum hydrocarbons, and a balanced diet will promote recovery from anemia. Supportive care to ensure adequate caloric and fluid intake, antibiotic therapy to control infections, and vitamin and mineral supplementation are recommended. Vitamin B-complex should be given to promote erythrocyte maturation. Because iron is readily taken up by macrophages and sequestered in monocyte-macrophage pools during chronic inflammation, serum iron may be low in anemic animals. Supplemental iron preparations (ferrous sulfate at 0.2 g/ day PO for at least two weeks) are beneficial as long as infections are controlled. Androgenicanabolic steroids (stanozolol, 10-25 mg/ otter per week IM) stimulate red blood cell production and may be useful in treating various forms of anemia. However, it may take weeks or months for the hematopoietic effect of steroid treatment to become apparent and it is contraindicated for gravid females. Weekly blood samples should be taken to monitor packed cell volume.
In severe cases of anemia, blood transfusions may be considered. During the EVOS, this treatment was effective in correcting anemia in oiled birds (White, 1991). Blood transfusions assume an appropriate clinical setting and an adequate donor, two factors that are difficult to achieve for sea otters involved in an oil spill. Therefore, it is unlikely that transfusions will be a viable treatment for anemia in large numbers of oiled sea otters.
Stress
(a) Etiology. Stress is a term used to describe the psychoendocrine response of an animal to environmental and psychological stimuli that cause physical or mental tension. The hallmark of stress is activation of the pituitary-adrenal system, which may eventually result in adrenal hypertrophy, thymicolymphatic involution, gastric ulceration, and suppression of testicular and ovarian function (Levine, 1985). Although the complexity of the stress response makes it difficult to diagnose and monitor, it is apparent that stress will hinder recovery from oil exposure in wild animals.
Oiled otters brought to rehabilitation centers are subject to many strange and unfamiliar situations that may cause stress. The rehabilitation process, from capture and cleaning to treatment and release, can be stressful for wild otters. Manifestations of stress often can not be differentiated from the detrimental effects of oil exposure. However, 25% of the lightly oiled and unoiled otters brought to rehabilitation centers during the EVOS died (Chapter 1). Stress associated with the rehabilitation process may have contributed to this mortality. Also, stress associated with capture and rehabilitation may reduce an animal’s resistance to diseases which occur naturally in the wild population (Harris et al., 1990).
Stress-induced immunosuppression in captive sea otters may result in prolonged wound healing and abnormal inflammatory responses to abrasions, cuts, and injections. White blood cell counts (WBC) vary widely for oiled sea otters (Appendix 3, Figure H Download PDF). Because their immune system may be compromised, we recommend the prophylactic administration of antibiotics on admission to rehabilitation centers (see Chapter 4). Elective surgical procedures should be delayed until leukograms and serum chemistry panels return to normal.
(b) Clinical Manifestations and Diagnosis. Stressed sea otters may vocalize continuously, lack locomotor coordination, become anorectic, and exhibit stereotypic behaviors such as prolonged grooming and fur chewing. Plasma catecholamines and corticosteroids may increase initially, then diminish as the otters habituate to the rehabilitation process. However, some otters are less adaptable and may never habituate to captivity. Many of the otters that died in the rehabilitation centers during the EVOS had gastric ulcers and adrenal abnormalities suggestive of stress (Chapter 1; Lipscomb et al., 1993, 1994).
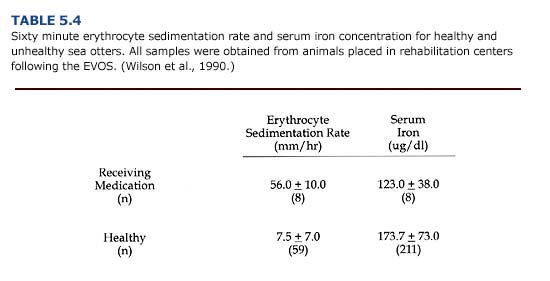
Erythrocyte sedimentation rate (ESR) and serum iron are useful indicators of some types of stress (Table 5.4). The red blood cell sedimentation rate increases for animals with infections and inflammation. Blood samples from clinically ill otters during the EVOS had sedimentation rates seven times the normal rate. Serum iron decreased in response to bacterial infection and was lower in unhealthy otters.
The accumulation of lactic acid in the muscles of otters that are captured with dip nets has been associated with cases of capture myopathy syndrome (Williams and VanBlaricom, 1989). During prolonged chases, increased muscle lactic acid concentration can cause serious damage to muscle fibers. Elevations in both creatine phosphokinase (CPK greater than 490 IV/I) and lactate dehydrogenase (LDH greater than 419 IV/I) indicate skeletal muscle damage and were apparent for oiled otters (Appendix 3, Figure F Download PDF).
(c) Treatment. Stress in sea otters at rehabilitation centers may be prevented by creating a predictable environment that reduces novelty. Good nutrition, access to seawater and haulout space, good sanitation and disease prevention, a tolerable thermal environment, a sense of safety, and opportunities to socialize with other otters are important environmental elements. Most of these requirements can be achieved with properly designed facilities, good husbandry and veterinary care, and by minimizing physical contact between the staff and otters (see Chapter 7 and Chapter 12).
Diazepam (0.2 mg/kg PO or 0.1 mg/kg 1M) may alleviate destructive behavioral responses to stress such as excessive grooming, fur and skin biting, and anorexia. Treatment with diazepam allows the animal to slowly regain normal patterns of behavior that facilitate recovery. Also, low dosages of diazepam may stimulate the otter’s appetite, which will promote recovery.
Prevention is the key to managing capture myopathy syndrome. Any sea otter that eludes easy capture with a dip net is probably not suffering from the detrimental effects of oil exposure. Prolonged chases can cause serious elevations in tissue lactic acid concentration and consequent muscle damage. An alternative form of capture, such as a tangle net or Wilson trap, should be used for these otters. Throughout the capture and rehabilitation process, physical stress should be minimized. For otters with suspected capture myopathy syndrome, supplemental vitamin E (400 IV/day PO) and selenium (SeletocTM, 0.1 rnI/kg as a single dose IM or SQ in two sites) are recommended.

