The period of stabilization begins at the moment of capture and ends when the animal is ready for cleaning at the rehabilitation center. The goal of stabilization is to correct immediate life-threatening conditions (i.e. hypothermia, hyperthermia, hypoglycemia, shock, severe dehydration, sepsis) so that the otter can tolerate stresses associated with transport, handling, and cleaning. Heavily oiled sea otters should be cleaned as soon as they are clinically stable to minimize further absorption of oil. Cleaning may be postponed twenty-four hours for otters that are lightly oiled and have no serious clinical disorders. Criteria for determining the duration of a stabilization period are presented in Chapter 11.
All sea otters should receive a physical examination as soon as possible after capture. A veterinarian or animal care specialist should diagnose and treat symptoms that are immediately life-threatening. Oiled sea otters may exhibit signs of hyperthermia or hypothermia, dehydration, shock, lethargy, seizures, and depression. Respiratory and cardiovascular function should be evaluated and stabilized first, with subsequent treatments dependent on alleviating the underlying cause of the dysfunction. The normal heart rate of adult sea otters is 144-159 beats/minute, but the average heart rate can increase to 199 beats/ minute during agitated grooming (T. M. Williams, unpublished data). Respiratory rate ranges from 17-20 breaths/minute for adult sea otters (Appendix 1 Download PDF).
Initial Assessment Parameters
Along with heart rate and respiratory rate, the following parameters should be assessed immediately for otters arriving at a rehabilitation center. Most can be determined very quickly by palpation or visual observation.
(a) General Body Condition. Oiled otters may not eat in the wild, and therefore may be dehydrated and underweight. Normal body weights for adult Alaskan sea otters range from 27-48 kg for males and 16-32 kg for females. California sea otters are slightly smaller. Oiled otters often exhibit symptoms of hypoglycemia, including depression, seizures, muscular weakness, and hypothermia. A naturally high metabolic rate makes otters susceptible to hypoglycemia when deprived of food for more than several hours. To avoid or mitigate hypoglycemia and dehydration, food and ice should be offered to sea otters at least every three hours, except when they are asleep at night. Food and fluids should be withheld from otters for one hour before sedation to prevent vomiting and aspiration.
(b) Activity Level. The responsiveness of oiled sea otters can range from agitated to lethargic, and will depend on the duration and degree of exposure to oil. Early in a spill, the oil may be irritating to the skin and sensitive membranes around the eyes, nose, and flippers. In such instances, the otter may scratch its cornea and the membranes surrounding the eyes, or chew on the interdigital webbing of the hind flippers. In severe cases, cartilage on the edge of the ears or between the toes will be exposed. Excessive grooming will damage the fur by promoting hair breakage and shedding. With reduced levels of contamination, the otters will usually remain alert, groom, and accept food. Normal grooming behavior includes rubbing the ears, muzzle, and forearms, as well as licking and nuzzling the abdomen.
(c) Body Temperature. Oiled sea otters are thermally unstable and may be hypothermic or hyperthermic. If the animal is lethargic or unconscious, its core body temperature should be measured using an electronic digital thermometer with a flexible probe inserted fifteen cm into the rectum. Normal core temperature for sea otters ranges from 37-39°C (98.6-102°F). If the flexible probe cannot be easily inserted into the rectum, abnormally low or high core temperatures can be qualitatively verified by feeling the hind flippers and by observing behavioral signs. Shivering may be indicative of hypothermia, while panting and flipper expansion are commonly observed for hyperthermic otters. A hypothermic otter (core temperature less than 35°C or 95°F) will have cold hind flippers. In severe cases, the animal may be unconscious. Treatments for mild hypothermia during the stabilization period should be limited to placing the animal in a well-ventilated, warm (20°C or 68°F) area and drying the fur vigorously with towels and a pet dryer (set at room temperature). More aggressive treatments for hypothermia should be conducted under the controlled conditions of the rehabilitation center (see Chapter 5). Hyperthermic otters (core temperature greater than 40°C or 104°F) will have hot hind flippers, pant, and may exhibit agitated behavior. In severe cases, the overheated otter will be lethargic or unconscious. Chipped ice placed in the bottom of the cage will help cool hyperthermic otters awaiting cleaning.
(d) Coat Condition. Degree of oiling and water repellency should be assessed (see below and Chapter 6). Normal pelage will have a brown striated appearance. The underfur of the healthy coat remains dry even after submergence.
(e) Hydration. Exposure to crude oil is known to contribute to dehydration in marine mammals, often as a result of gastrointestinal disturbances (St. Aubin, 1990). Because 50-100% of the water intake of sea otters is derived from food (Costa, 1982), the inability to feed will lead to dehydration. Dehydration may be detected through physical examination by decreased skin elasticity, sunken globes, and dry mucous membranes. If dehydration is diagnosed or suspected, prophylactic fluid therapy is recommended. Normal saline or a 1-to-1 mixture of 5% dextrose solution and normal saline (20 ml/kg/ day SQ or IV) should be given.
(f) Signs of Pulmonary Distress (diaphragmatic breathing, hyperventilation, congestion). Following exposure to oil, the animals may show labored breathing and congestion associated with emphysema and inflamed nasal, pharyngeal, and bronchial membranes. Nasal discharges should be noted.
(g) Evidence of Shock. Signs of shock include muscle weakness, hyperventilation, cold hind flippers, pale coloration or mottling of the gums, and reduced capillary refill time following compression.
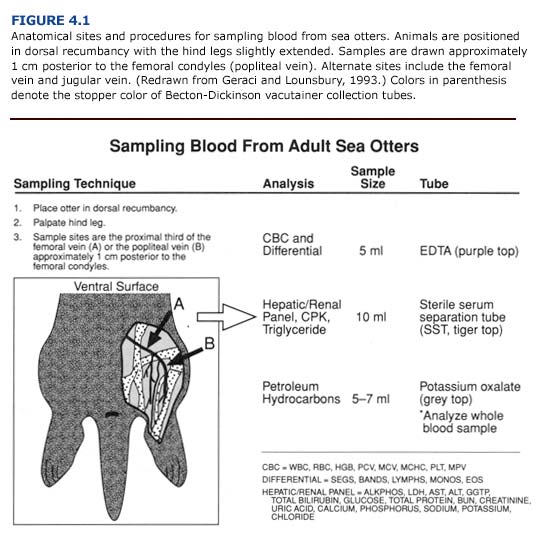
Following the general examination, blood samples from the femoral, jugular, or popliteal veins should be taken before cleaning or treatment (Figure 4.1). Blood glucose should be measured immediately using reagent strips (Chem StripsTM, BG Boehringer Mannheim, Indianapolis, Ind.), a desktop analyzer, or diagnostic units designed for at-home use by diabetics. Basic hematological and blood chemical constituents (glucose, blood urea nitrogen, hematocrit, erythrocyte sedimentation rate, white and red cell counts) are easily assessed with manual techniques utilizing desktop blood analyzers (Eastman Kodak, Inc.; Abbot Laboratories). These parameters provide rapid biochemical profiles which should be determined at the rehabilitation center to provide immediate diagnostic information for the veterinary staff. Comprehensive blood panels may be obtained later by sending the remainder of the blood sample to a veterinary diagnostic laboratory or appropriate facility with automated diagnostic equipment.
Results from the initial clinical evaluation should be entered on admissions forms (Appendix 2, Forms F and H) which will remain with the animal throughout the rehabilitation process. Data recorded on the forms will be used in rating each animal during triage and will provide the basis for subsequent treatments.
Treatments during stabilization should include the prophylacti administration of:
1) enrofloxacin (2.5 mg/kg bid IM or PO) for mature animals and amoxicillin (12 mg/kg bid 1M) for immature otters to prevent or treat infections,
2) dexamethasone (1-2 mg/kg/day IM or IV) to prevent or treat shock,
3) vitamin and mineral supplements including vitamin E (400 IU/day), vitamin B-complex, and selenium (SeletocTM, 0.1 ml/kg single dose IM or SQ in two sites). These supplements can be given as a multivitamin tablet (SeaTabsTM),
4) cimetidine (5-10 mg/kg tid PO or 10 mg/kg qid IV or IM) or ranitidine (1-4 mg/kg tid PO) for gastric ulcers, and
5) diazepam (0.2 mg/kg PO or 0.1 mg/kg IM) to reduce stress and stimulate appetite. This treatment is optional.

