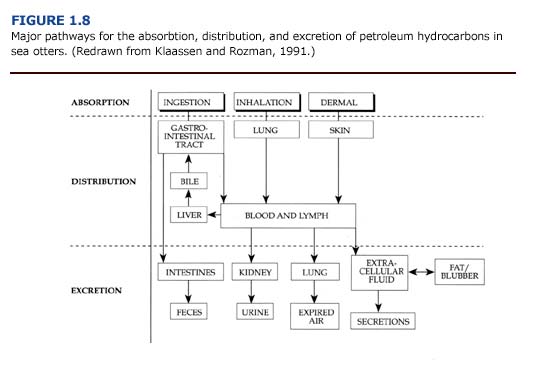
The absorption, distribution, and excretion of petroleum hydrocarbons involve many organ systems (Figure 1.8). Primary routes of exposure to petroleum hydrocarbons are ingestion, inhalation, and dermal absorption involving the gastrointestinal system, lungs, and skin, respectively. The liver and kidneys have a high capacity for binding toxicants and are considered major sites of toxicant concentration and accumulation (Klaassen and Rozman, 1991). Enzymes for the biotransformation of toxicants are located in these organs as well as the lungs and intestine (Sipes and Gandolfi, 1991). Petroleum hydrocarbons may be excreted through feces, urine, expired air, and, to a lesser extent, other secretions. Alternatively, petroleum hydrocarbons may be stored, and hence accumulate, in lipid rich tissues such as fat or blubber.
Macroscopic and microscopic evidence suggests that in contaminated sea otters, the liver, kidneys, gastrointestinal tract, and lungs are particularly vulnerable to oil, damage. Of the organ systems examined, the lungs had the highest incidence of lesions. Nearly 70% of the otters that arrived at rehabilitation centers during the first two weeks of the spill showed evidence of pulmonary damage (Williams et al., 1990). Other gross lesions included: 1) liver abnormalities in 55% of adult and juvenile otters, 2) gastrointestinal lesions in more than 50% of the otters, 3) cardiac lesions in 42% of the animals, and 4) kidney and spleen abnormalities in approximately 20% of the sea otters. Similar patterns are described for oiled sea otters by Lipscomb et al. (1994) and Mulcahy and Ballachey (1994). Specific lesions observed in sea otters during the EVOS are described below for each major organ system.
Liver
(a) Necropsy Observations. Because of its central role in metabolism, detoxification, and excretion of foreign chemicals, the liver is more likely to be exposed to petroleum hydrocarbons and their metabolites than other organs. Livers from oiled otters had a high incidence of lesions. Gross abnormalities included abnormal color and texture, enlarged lobes with prominent rounded edges, and atrophy. Livers were often friable and pale yellowish or reddish-brown in color with pale green mottling. There was no correlation between the degree of external oiling and the incidence of these lesions. Almost 75% of the unoiled animals examined had liver congestion, hemorrhage, necrosis, friable texture, or discoloration.
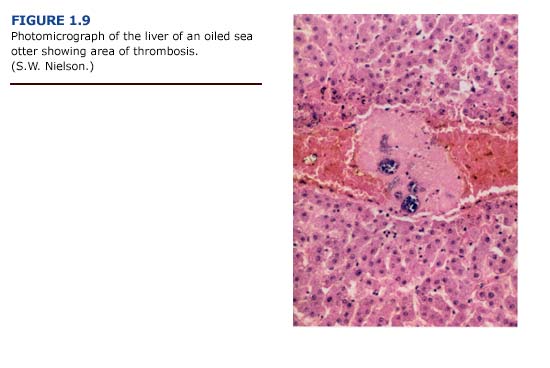
(b) Histopathology. Lesions of the liver were more impressive histologically than grossly. The histologic changes included congestion, hemorrhage, and a variety of degenerative changes such as hydropic degeneration, fat accumulation, and centrilobular necrosis. Necrosis was occasionally severe, with confluence of adjacent lobules to form larger areas. Generally, the centrilobular areas showed the most damage (Figure 1.9, see plate facing page 12). Periportal to diffuse hepatic lipidosis has been reported previously in oiled sea otters from the EVOS (Lipscomb et al., 1993, 1994).
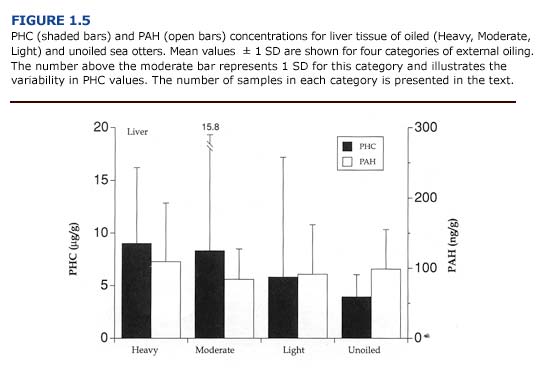
(c) Toxicology. Of the tissues examined, the liver generally contained the highest concentrations of PHC and PAH (PHC range = 0-36.4 ug/ g; PAH range = 0-234.9 ng/g; n = 21 oiled and unoiled animals). The levels of petroleum hydrocarbons in the liver did not correlate with the severity of external oiling or histologic lesions (Figure 1.5). For example, the liver from one heavily oiled animal with high concentrations of PAH and PHC appeared normal, with the exception of minor discoloration. Histopathological findings for this animal were also minor and consisted of periportal fatty change. In contrast, marked- to-severe congestion and necrosis of the liver was often accompanied by undetectable-to-moderate accumulation of petroleum hydrocarbons in other oiled and unoiled animals.
(d) Clinical Pathology. Blood chemistry panels indicated hepatic dysfunction for many of the oiled otters that died (Appendix 3, Figure E Download PDF). Plasma concentrations of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were elevated in these animals. Also, oiled otters were often hypoglycemic, although this may have been caused by many factors. (See Chapter 5.)
(e) Summary. The pattern of liver necrosis and congestion in oiled otters is compatible with both cardiovascular and toxicological damage. Cellular damage was often located in centrilobular rather than periportal areas (Figure 1.9). We found thrombi formation leading to vessel occlusion and local congestion in the livers. As a result, the centrilobular hepatocytes, which are located furthest from oxygen- carrying blood vessels, were the most likely to become hypoxic and necrotic. This pattern was evident in the majority of sea otters regardless of the degree of external oiling or level of petroleum hydrocarbon accumulation. Shock and hypothermia are the probable causes of the cardiovascular insufficiency which led to these patterns of liver damage. These findings suggest that liver damage and dysfunction in oiled sea otters results primarily from vascular congestion with systemic petroleum hydrocarbon toxicity playing a secondary role.
Kidneys
(a) Necropsy Observations. Macroscopic examination of the kidneys revealed few abnormalities in oiled and unoiled sea otters. Cortical streaking, pale coloration, and mild congestion were occasionally noted, but these did not correlate with the degree of external oiling or histologic findings.
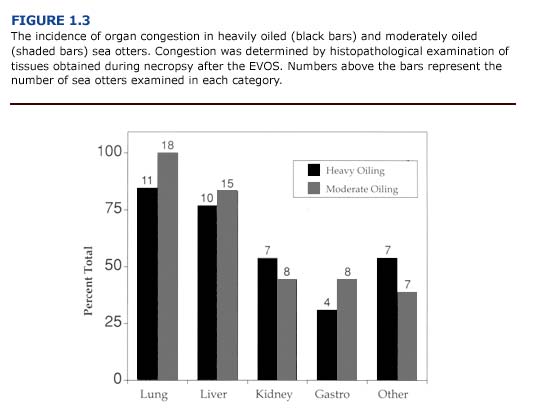
(b) Histopathology. In contrast to the macroscopic findings, the microscopic renal lesions correlated well with the degree of external oiling. Kidneys from heavily and moderately oiled otters typically showed moderate to severe levels of congestion (Figure 1.3). Severe to moderate tubular lipidosis was commonly observed in these animals (present study, Lipscomb et al., 1993, 1994). Fewer remarkable lesions were observed in otters with less severe degrees of oiling.
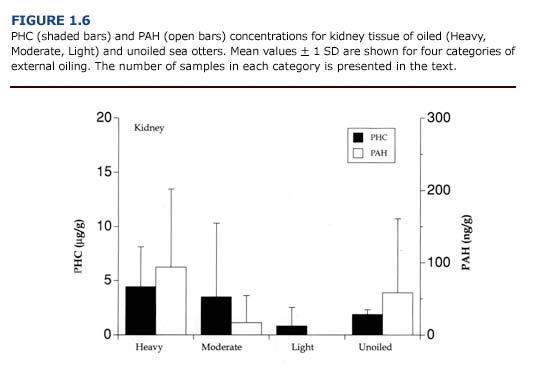
(c) Toxicology. PHC and PAH levels in the kidneys (Figure 1.6) were generally lower than reported for the liver (Figure 1.5). As observed for the liver, there was no apparent correlation between the concentration of petroleum hydrocarbons in the kidneys and the degree of external oiling. Likewise, the level of petroleum hydrocarbons did not always correspond to histopathological findings. Petroleum hydrocarbons appear to be cleared rapidly from the sea otter kidney. Samples of kidney from oiled otters that survived at least eleven days showed no evidence of petroleum hydrocarbon accumulation regardless of the degree of external oiling.
(d) Clinical Pathology. Acute renal insufficiency was evident for oiled sea otters and was manifested as elevations in serum concentrations of blood urea nitrogen (BUN), phosphorus (Appendix 3, Figures C, D Download PDF ) and creatinine. A complete description of this condition is provided in Chapter 5.
(e) Summary. Despite the importance of the kidneys as a site for biotransformation and excretion of toxicants, there was little accumulation of PAH or PHC in this tissue. Renal lipidosis was found only in otters that had hepatic lipidosis (Lipscomb et al., 1993). Many factors including infection, petroleum hydrocarbon exposure, mobilization of fat reserves during periods of reduced caloric intake, and hypoxia induced by vascular collapse may have contributed to this condition.

Lungs
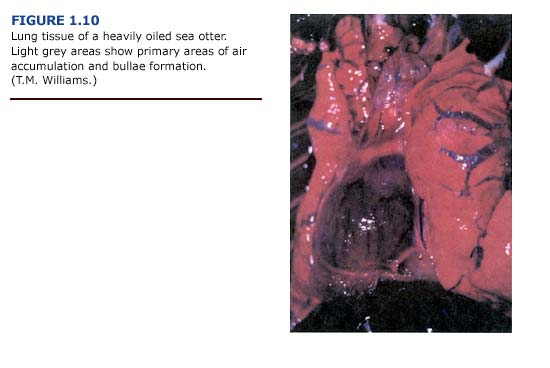
(a) Necropsy Observations. One of the most common lesions observed in otters captured during the first three weeks of the EVOS was bullous interstitial emphysema, which was characterized by the accumulation of interlobular air bubbles. In its most severe form, air was trapped along the trachea, mediastinum, and subcutaneous areas of the thorax. Emphysematous areas were patchy in distribution with no apparent preferential site. They ranged in size from a few millimeters to bullae formation of several centimeters in diameter (Figure 1.10, see plate facing page 12). The lungs typically appeared normal in color. Cases of subcutaneous emphysema were generally present only in animals with the most severe bullae formation in the lungs. The incidence and severity of emphysema generally correlated with the level of external oiling. Heavily oiled adult animals showed gross or histologic evidence of moderate to severe emphysema, usually involving interstitial tissues. In contrast, only mild forms of emphysema were noted for thirteen lightly oiled adult otters. Emphysema was also observed in one otter that was apparently unoiled but had sustained traumatic injuries during a fall. Depending on the severity, subcutaneous emphysema was detectable by palpation and correlated with macroscopic lesions observed during necropsy.
(b) Histopathology. Although histopathological examination of lung tissues demonstrated focal areas of congestion, edema, and alveolar distension, there was little indication of alveolar emphysema characteristic of chronic emphysema in humans. The histologic alveolar changes were fairly mild and subtle despite the incidence of severe interstitial and subpleural emphysema with bullae formation. There was no morphological evidence of damage to the airways or alveolar walls. Subpleural, interstitial, or subcutaneous bullous emphysema, rather than pulmonary or alveolar emphysema per se, are more appropriate terms for the conditions observed in oiled sea otters.
Pneumonia induced by the aspiration of oil, a characteristic respiratory lesion of petrochemical poisonings (Hatch, 1988), was not apparent in oiled sea otters during the EVOS.
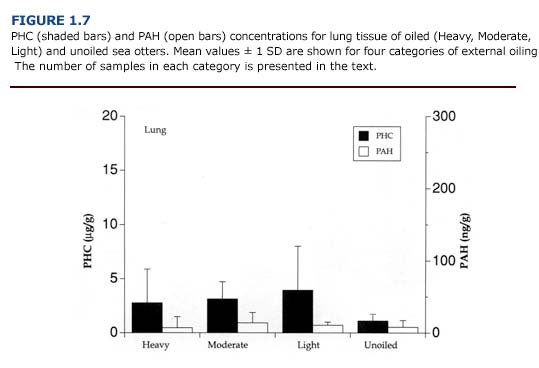
(c) Toxicology. The levels of PAH and PHC were comparatively low in the lungs (Figure 1.7). Mean PHC levels in oiled otters were 31-68% of the mean levels for liver. PAH concentrations were less than 20% of the values for liver samples. Unlike the kidneys and liver, there was a good correlation between petroleum hydrocarbon accumulation and tissue injury (i.e. emphysema). The average hydrocarbon concentrations in the lungs of otters showing severe congestion or emphysema were 3.68 %B1 3.19 ug/g for PHC and 21.14 %B1 14.01 ng/g for PAH (n = 5). In animals with mild or moderate congestion or emphysema, PHC levels were 1.42 %B1 1.00 ug/ g and PAH concentrations were 4.13 %B1 5.45 ng/ g (n = 9). Likewise, circulating levels of paraffinic hydrocarbons correlated well with the severity of emphysema (Chapter 4).
(d) Clinical Pathology. None of the standard tests for hematology or blood chemistry provided insight into lung damage. In the future, the partial pressures and concentrations of blood gases may prove useful in assessing the severity of respiratory injury in oiled sea otters.
(e) Summary. The pathogenesis of emphysema in oiled otters is not understood. Contributing factors may include weakening of the respiratory mucosa by petroleum hydrocarbon vapors and mechanical damage associated with respiratory exertion during capture, diving, coughing or agonal death. Damage associated with exposure to oil is supported in part by the positive correlation between blood and tissue petroleum hydrocarbon concentrations and the severity of emphysema. Because the incidence of emphysema was greatest during the first three weeks of the spill, exposure to the volatile aromatic hydrocarbons in fresh crude oil may have contributed to the development of the lesions. Twenty-five of forty-one cases of emphysema occurred within fourteen days of the EVOS. Only two instances of severe bullous interstitial emphysema were recorded after the first three weeks of the spill. Histopathological findings provide little insight into the pathogenesis of the lung lesions. Bullous emphysema has not been previously reported as a major injury in oiled marine mammals, except for a brief reference to the condition in one of four experimentally oiled polar bears (Oritsland et al., 1981).
Ventilatory exertion has been implicated in the development of spontaneous subcutaneous cervical and mediastinal emphysema in humans (Parker et al., 1990). Similarly, altered respiratory mechanics may also contribute to respiratory injury in oiled sea otters. Irritation of bronchial airways by volatile petroleum hydrocarbons may cause bronchial constriction. Extraordinary ventilatory exertion during such spasms could result in pulmonary distension and induce bullae formation. Predive inspiration, exercise-induced hyperventilation, extraordinary inspiratory effort due to blocked nares, and agonal breathing at death also may cause airway distension. Most likely, the cause of emphysema in oiled sea otters is a combination of chemical factors such as exposure to fresh or irritating crude oil, and mechanical factors including forced ventilatory expiration coupled with bronchial constriction.
Gastrointestinal System
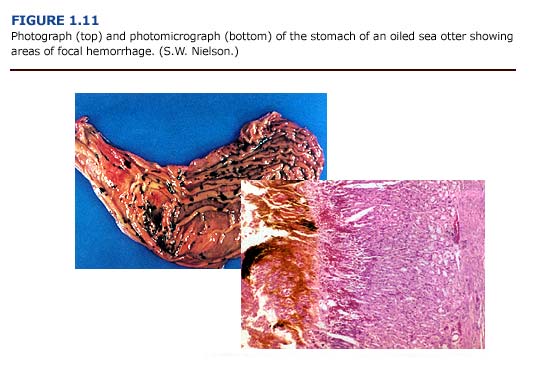
(a) Necropsy Observations. Gastrointestinal lesions were observed in sea otters throughout the rehabilitation process. The most frequently observed gastrointestinal lesions were areas of focal hemorrhage, ulceration, inflammation, and parasitism (Figure 1.11, see plates facing page 13). Gastrointestinal mucosal ulcerations, primarily involving the pylorus and the proximal small intestine, were a common finding. The consistency of the gastric and intestinal contents varied from a watery to a viscous dark brownish-green to reddish-black fluid. Several animals had dark black, tarry intestinal contents, although tests were not conducted to confirm the presence of oil or blood in the feces. In a related study, Mulcahy and Ballachy (1993, 1994) reported petroleum hydrocarbon levels indicative of oil ingestion in three of ten otters examined from the EVOS. Intestinal parasitism, primarily due to acanthocephalans, was a common finding. Cestode and nematode infestation frequently occurred. Ulceration of the oropharyngeal mucosa, especially the gingival mucosa, was observed, and intestinal intussusception occurred in several animals.
(b) Histopathology. Because of the focal nature of these lesions, histopathologic observations did not always correspond to macroscopic reports. Thus, a higher incidence of ulceration was reported during postmortem examinations than from the histopathology. Gastrointestinal lesions were found in both unoiled and oiled otters. The gastric mucosa showed acute focal changes; these ranged from necrosis and ulceration to acute hemorrhaging with free hemolyzed blood in the intestinal lumen.
(c) Toxicology. No toxicological tests were conducted on tissues from the gastrointestinal tract. In the future, petroleum hydrocarbon analyses of stomach contents and bile could prove valuable by providing information about the ingestion and excretion of crude oil.
(d) Clinical Pathology. Anemia may have been a secondary effect of gastrointestinal hemorrhaging. This is discussed in Chapter 5.
(e) Summary. It is difficult to relate the degree of external oil contamination to gastrointestinal abnormalities in oiled sea otters. There was a positive correlation between the degree of oiling and the incidence of stomach ulcers, but not for intestinal ulcers (Williams and Davis, 1990). Gastrointestinal hemorrhaging and superficial ulceration of the stomach mucosa may have been caused by shock, stress, or hypothermia (Paton, 1991). Stress induced gastrointestinal hemorrhaging is common in many mustelid species, including otters. In tests with laboratory animals, many components of crude oils are not considered ulcerogenic (Beck et al., 1984). Therefore, it is unlikely that petroleum hydrocarbon exposure alone would cause the degree of gastric erosion observed. Because lesions were evident throughout the rehabilitation process, stress, hypothermia, shock, parasite infestation, and oil contamination are all considered contributing factors of gastrointestinal lesions in oiled sea otters.
Other Organs and Tissues
Adrenal hyperplasia, immunosuppression, and anemia have been identified as potential problems for oiled wildlife placed in rehabilitation centers (Leighton, 1991; White, 1991). The cause of these problems has been attributed to the combined effects of stress and oil toxicosis.
Adrenal hyperplasia, one manifestation of chronic stress, could not be confirmed macroscopically or microscopically in sea otters. Although enlarged adrenals were frequently noted at necropsy, that they were not weighed prevented a quantitative comparison with normal glands. However, hyperplasia of adrenal tissue was noted histologically for six of the animals examined. The incidence of hyperplasia did not correlate with the duration of captivity or degree of oiling. Histological examination showed that 60% of the otters (n = 32 animals) had no remarkable adrenal lesions.
Immunosuppression may be caused by a variety of petrochemicals. The polycyclic aromatic hydrocarbons (PAHs) in particular may produce a marked and prolonged depression in both primary and secondary serum antibody responses (Dean and Murray, 1991). Indirect evidence of immunosuppression has been observed for oiled polar bears (Oritsland et al., 1981) and oiled sea otters (Williams and Davis, 1990). Indications included abnormal inflammatory responses to sterile injections and to routine cuts and abrasions. Lesions were observed in the lymph nodes of approximately 45% of the heavily and moderately oiled sea otters. Moderate atrophy, histiocytosis, congestion, and hemorrhaging of lymph tissues were reported. The mechanisms by which immunosuppression develop are numerous and difficult to assess based on hematology; white blood cell counts were highly variable for all sea otters. Detailed examination of the cellular components of the bone marrow were not conducted. Although immunosuppression may occur in oiled sea otters, further research is needed to determine the incidence and the severity of the problem. Many factors, including exposure to petroleum hydrocarbons, hypothermia, stress, nutritional changes, and captivity, may challenge the immune systems of oiled animals during rehabilitation.
Anemia occurred frequently. Heavily and moderately oiled otters showed a higher incidence of anemia than lightly oiled animals. In general, the packed cell volume (PCV) of newly captured otters was within the normal range of 40-66% for healthy adult sea otters (Thomas Williams, in press); the values did not correlate with the degree of oiling or survivorship. Decreases in PCV occurred after several weeks in captivity, with a return to normal levels within four months (Williams, 1990). (See Chapter 5 for a discussion on anemia in oiled sea otters.)

