The composition and toxicity of crude oil changes as it degrades following a spill. The rate of degradation depends on ambient temperature and ocean conditions, with fresh crude oil often remaining toxic for approximately three to seven days (Neff, 1990). 1990). In sea otters, the internal and external consequences of contamination are different for fresh and weathered oil (Williams, et al.,1988; Williams and Davis, 19990). Consequently, the condition of oiled otters may vary over the course of an oil spill. From the perspective of wildlife, it is helpful to subdivide catastrophic oil spills into two phases; an Early Phase comprising the first two to three weeks of most spills, and a Late Phase consisting of the remainder of the clean up effort or rehabilitation program. During the Early Phase, the oil contains the greatest concentration of aromatic petroleum compounds (volatiles) and is considered the most toxic. Animals which arrive at a rehabilitation center during this period will show the highest incidence and severity of medical problems (Williams, 1990). During the Late Phase, the number of animals requiring rehabilitation will diminish. These animals also show jess external oiling and fewer medical conditions.
The division between Early and Late Phases of an oil spill is less distinct for chronic events involving long term oil release such as an oil platform blowout or leaking transport vessel. During these events, wildlife responses to contamination will depend on the composition of the oil encountered, degree of weathering, and the duration of exposure.
The initial assessment of oil contamination in sea otters is made by visual examination of the pelage. Four classifications are suggested:
1) heavily oiled (>60% body coverage with saturation to the skin),
2) moderately oiled (30-60% body coverage that includes areas of saturation),
3) lightly oiled <30% body coverage or light sheen on fur), or
4) unoiled (no visual or olfactory evidence of oiling).
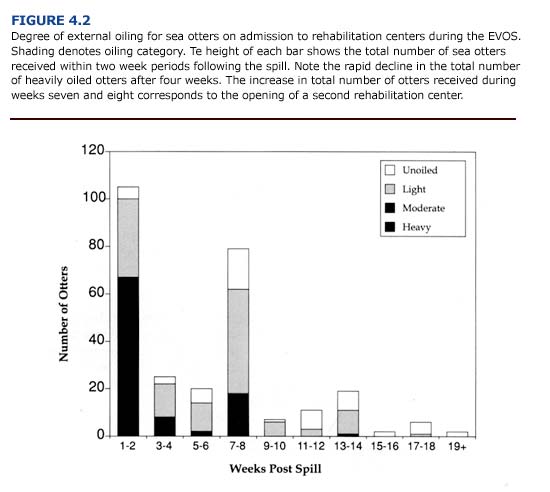
The contamination level in sea otters will change as the oil composition changes. For example, Figure 4.2 shows external contamination that occurred in sea otters during the Exxon Valdez oil spill (EVOS) (Williams et al., 1990). Almost 60% of the otters arriving at rehabilitation centers during the first two weeks of the spill were heavily oiled. By the fourth week, the majority of otters were lightly oiled. As the rehabilitation program continued, the number of otters arriving at rehabilitation centers and the degree of oiling decreased rapidly with time. While ninety-four otters were retrieved in the first two weeks, only forty-seven animals arrived during weeks three and four. Two months after the spill, less than one otter arrived per day at rehabilitation centers.
As the oil becomes more diffuse, detection on the fur becomes increasingly difficult. Sheen oil, in particular, is difficult to detect on sea otter fur. A noticeable petroleum odor or stickiness of the fur indicates contact with oil.
Internal exposure to petroleum hydrocarbons is comparatively more difficult to verify. Furthermore, the toxicity of ingested oil is not fully known. A quick, relative measure of systemic exposure for large numbers of animals may be obtained by measuring petroleum hydrocarbon levels in blood samples. The concentration of petroleum hydrocarbons provides important diagnostic information, if the sample is obtained within forty-eight hours of exposure to oil. Because some petroleum compounds may be cleared quickly from the blood, longer delays between exposure and blood sampling may lead to false negative results. To avoid lengthy, difficult, and expensive analyses, we suggest measuring total paraffinic hydrocarbons, rather than the more toxic polycyclic aromatic hydrocarbon (PAH) compounds (Neff, 1990). If a blood sample is taken soon after exposure to oil, paraffinic hydrocarbon levels for individual samples are proportional to PAH concentrations. This test was used during the EVOS and is recommended for assessing petroleum hydrocarbon exposure in oiled otters.
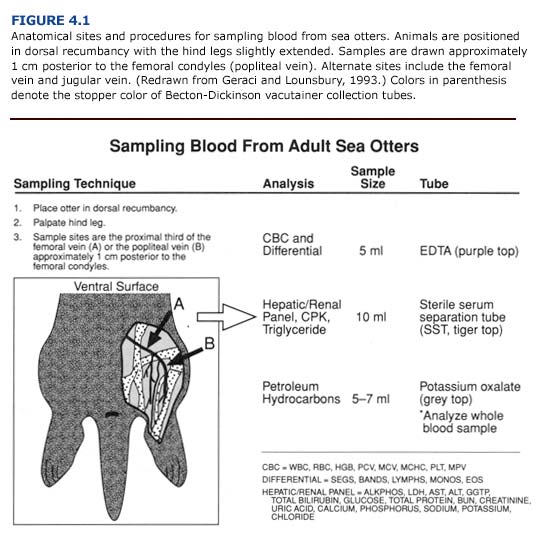
Five ml blood samples are drawn from the femoral, jugular, or popliteal veins (Figure 4.1) and placed in potassium oxalate vacutainers (Becton-Dickinson). Whole blood samples should be immediately transferred to acid washed vials, frozen, and stored at -10°C until analysis. National Medical Services, Inc. (Willow Grove, PA) and the Geochemical and Environmental Research Group of Texas A&M University (College Station, TX) conduct tests for petroleum hydrocarbons in blood samples (see Chapter I, Table 1.2). Local hospitals may suggest additional analytical facilities near the site of the rehabilitation center. The choice will depend on cost, shipping time, and the laboratory analysis time. To provide the most benefit to the otters and attending veterinarians, test results should be available within two to three days of blood sampling.

The recommended analysis provides the veterinarian with a value for the total concentration of paraffinic hydrocarbons (C3 -36) in each blood sample. Baseline levels of these paraffins, determined from unoiled adult Alaskan sea otters held in seaquariums, are, less than one ppm. Higher levels indicate acute exposure to petroleum hydrocarbons. The presence of paraffinic hydrocarbons in the blood is a signal for veterinarians to initiate more aggressive treatments for mitigating oil toxicosis. (See Chapter 5.)
Several trends were apparent following the analysis of paraffinic hydrocarbons in sea otters contaminated during the EVOS (Williams and Davis, 1990). First, total paraffinic hydrocarbon (TPH) concentrations in the blood were variable for oiled sea otters. TPH ranged from 19 ppm to 800 ppm in adult sea otters on admission to rehabilitation centers (Figure 4.3). Second, internal exposure based on TPH levels did not consistently correlate with the degree of external oiling. Rather, the primary correlation appeared to be between TPH concentration and when the animal was exposed to the oil (ie. during the Early or Late Phases of the spill).
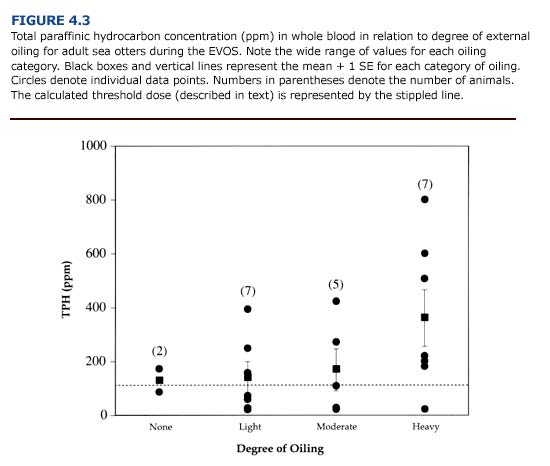
In view of this, the highest paraffinic hydrocarbon levels should be expected for heavily oiled animals captured during the first two weeks of a spill. The mean TPH concentrations for lightly and moderately oiled otters will probably be indistinguishable from one another. Low TPH levels can occur in animals from all four categories of oiling. In general, external contamination will be a poor indicator of internal contamination and should not be used as an index of systemic exposure.
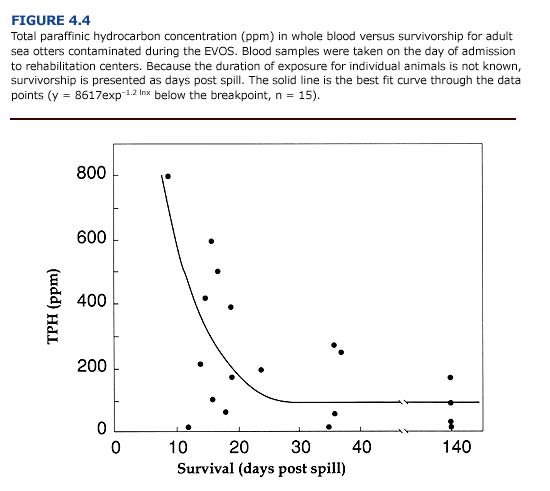
The likelihood that a contaminated animal will survive can be assessed from the threshold dose (TD) of the contaminant (Klaassen and Eaton, 1991). The TD for North Slope crude oil during the EVOS was calculated from the relationship between the survivorship of oiled otters and the concentration of paraffinic petroleum hydrocarbons in
the blood. In oiled sea otters, survivorship increased as TPH concentration decreased (Figure 4.4). Based on this curve and individual variation in TPH concentration for otters surviving at least twenty days after contamination, the mean TD for crude oil during the EVOS was 112±92 SD ppm. Animals showing blood TPH levels below this value are more likely to survive than those with higher values. Note that the highest level of TPH measured for an oiled sea otter that survived to release was 171 ppm.
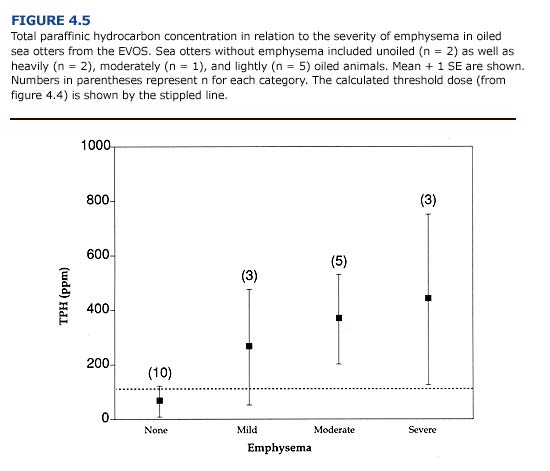
The threshold dose also provides a useful index of systemic damage in acutely oiled sea otters. This is demonstrated by examining the relationship between TPH concentration and the incidence of emphysema for otters contaminated during the EVOS (Figure 4.5). Blood TPH levels of otters displaying emphysema were significantly higher (at p<0.00l) than those for healthy otters. TPH levels also correlated well with the severity of emphysema. Sea otters without emphysema had a mean TPH level of 65±SE ppm (n = 10), well below the calculated threshold dose. All animals diagnosed with subcutaneous emphysema had TPH levels greater than 224 ppm, more than two times the mean threshold value of 112 ppm.
Although the TPH concentration provides little information about exposure to specific petroleum hydrocarbons, it provides a relative index of toxicosis. Lethal thresholds based on TPH concentration will depend on the type of oil encountered, duration of contact, and the species affected. Factors such as the origin and type of petroleum product and the state of weathering influence the petroleum hydrocarbon composition, and thus, toxicity of the oil. Acute exposure will yield different responses than chronic exposure. Furthermore, individual species may respond differently to oil contamination (see Chapter 15).

