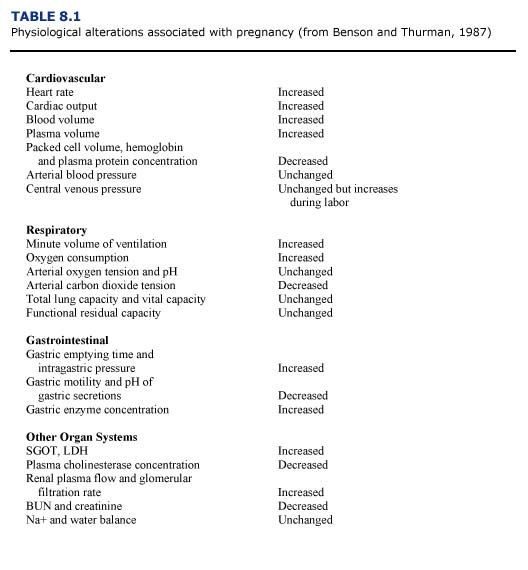
Data concerning the physiological changes occurring with gestation and parturition indicate an increased health risk for pregnant mammals during anesthesia (Table 8.1; Benson and Thurmon, 1987). For example, cardiac work is increased during pregnancy, thus creating a decreased cardiac reserve. The increase in plasma volume may be greater than increases in red blood cell mass, resulting in decreased hemoglobin concentration and packed cell volume (PCV) in the pregnant anima1. If these changes are exacerbated by Heinz body formation associated with petroleum hydrocarbon toxicosis, the pregnant female may become anemic. Struggling during restraint or anesthetic induction could result in pulmonary congestion and heart failure in pregnant animals. The functional residual capacity of the lungs is decreased by pressure on the diaphragm and abdominal organs by the gravid uterus. The pressure of the enlarged uterus on the diaphragm and major abdominal vessels can alter oxygenation and return blood flow especially in marine mammals whose natural buoyancy probably minimizes these effects in the aquatic environment. Hypoxemia and hypercapnia may occur if the animal does not breathe properly. The fetus may be adversely affected by abnormal maternal acid/base balance or by decreases in maternal blood pressure which may reduce blood flow to the placenta and cause fetal hypoxia. Vomiting and aspiration are also more likely to occur in the pregnant animal during anesthesia. Complications include asphyxiation and pneumonitis.
In view of the above complicating factors, there is no ideal anesthetic for pregnant sea otters. Primary consideration should be given to minimizing the time from induction to recovery, and minimizing the effects of depression on the cardiopulmonary and respiratory systems of the mother (Benson and Thurmon, 1987).
The narcotic, fentanyl, was the most commonly used anesthetic at sea otter rehabilitation centers during the EVOS. It was usually combined with diazepam (to reduce the occurrence of seizures) and a tranquilizer such as azaperone or acetylpromazine (see Chapter 3). This protocol had the advantage of rapid and reliable induction after intramuscular injection. In addition, naloxone could be used as a reversal agent after completion of washing or treatments. All of these agents can cross the placenta (Briggs et a1., 1983).
Opiates can cause fetal depression proportional to the degree of analgesia produced. Fetal elimination of opiates may take up to two to six days in some species. Because opiate antagonists such as naloxone also cross the placenta, initial reversal of this depression will occur. However, naloxone itself can cause mild neonatal depression. Because naloxone has a short duration of action, renarcotization from the fentanyl may occur once the naloxone is metabolized and excreted. All otters, and especially neonates delivered within two days of maternal anesthesia, should be monitored for recurring signs of narcosis. Supplemental naloxone should be administered, if indicated.
Phenothiazine tranquilizers, including acetylpromazine, may promote opiateinduced depression but add little to analgesia. They induce hypotension, respiratory depression, and thermal instability. In contrast to the opiates, the duration of action of these tranquilizers is long (lasting up to eight hours) and cannot be reversed. The use of these tranquilizers in pregnant animals should be limited to markedly apprehensive or excited females. The doses should be minimal to produce a calming effect, but not undue generalized depression.
When administered to pregnant animals, diazepam can produce lethargy, hypothermia, and hypotonus in the newborn. These effects are dose related and do not appear to cause severe problems when minimal doses are used (Benson and Thurmon, 1987). As a cautionary note, fetal deformities have been associated with the use of diazepam in the first trimester in human patients (Briggs et a1., 1983).
Inhalation anesthetics such as isoflurane and halothane readily cross the placenta, resulting in rapid fetal and maternal equilibrium. Consequently, a degree of depression is created in the fetus that is proportional to the depth of anesthesia in the mother. Deep levels of maternal anesthesia may cause maternal hypotension, decreased uterine blood flow and fetal acidosis. The same responses are observed in mothers suffering from circulatory collapse due to hypoglycemia, hypothermia, or shock. In view of this, the use of inhalation anesthetic agents in hypotensive pregnant otters should be minimized or avoided.
Sea otters in advanced pregnancy should be placed in lateral recumbency (on their side) during anesthesia to reduce pressure on the diaphragm and major vessels. Anaesthetic times should be kept short. Intravenous or subcutaneous fluids (normal saline or a 1-to-1 mixture of 5% dextrose solution and normal saline; 20 ml/kg/day) should be administered to maintain adequate blood pressure and perfusion. If I available, assisted ventilation or supplemental oxygen may be beneficial in severely depressed animals. However, positive pressure delivery systems are not recommended if interstitial or subcutaneous emphysema is suspected. (See Chapter 5.) Constant monitoring of body temperature is vital and methods to warm or cool the animals must be provided. Care must be exercised when moving anesthetized otters in advanced pregnancy to avoid abrupt rotational movements which might induce uterine torsion.

